We have substantiated our dermatological safety claims through 48 hours closed human patch tests1 under occlusion to evaluate potential skin irritation after contact with the products.
1 Safety dermatological study performed by Complife Group, a multi-certified third party providing microbiological and chemical-physical testing.
AIM OF THE STUDY
This study assesses the potential side effects (skin erythema, oedema, or other types of skin irritation) that may occur after applying the tested topical products—The Gentle Cleanser, The Power Serum, The Active Cream—to evaluate whether they are safe for consumer use.
The tested cosmetic product conforms to Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (recast) —Text with EEA relevance—and to its annexes.
SUBJECT
25 volunteers were recruited to take a part in the test in accordance with specific inclusion2 and non-inclusion3 criteria.
Participants are withdrawn if they do not follow the conditions of the Study Information Sheet that they receive after the recruitment or if they suffer any illness or accident or develop any condition during the study which could affect the outcome of the study.
Through patch application and 24 hours after patch removal volunteers must avoid situations or activity that could interfere with clinical evaluations:
→ Exposition to sun or solarium.
→ Sport activity.
→ Immersion in water or steam bath.
→ Chafing and mechanical or thermal stress in the area in which patch is/has been applied.
2 Inclusion criteria: Healthy female and male subjects; Subjects between 18 and 70 years old; Subjects informed about test purposes.
3 Non-inclusion criteria: Subjects who do not fit the inclusion criteria; Pregnant or breastfeeding women; Subjects with marks (for example tattoos, scars, burns) in the tested skin region, which might interfere with clinical evaluation; Subjects with dermatological problems in the test area; Subjects with medication that may affect skin response; Subjects undergoing pharmacological treatment (both locally or systemically); Subjects with history for contact dermatitis; Positive anamnesis for atopy.
SAMPLE PREPARATION AND APPLICATION
The product is applied as it is by using the Finn Chamber, an 8 mm diameter aluminium disk.
The Finn Chamber is fixed to the skin with a tape already been tested for its safety that ensure the occlusive application of the product. Applied quantity is sufficient to fill the chamber without overflowing from it when applied on the skin. The product is left in contact with the skin surface for 48 hours. The cutaneous reactions are analysed at 15 minutes, one hour and 24 hours after Finn Chamber removal. A Finn Chamber containing a blotting paper disk soaked with demineralized water is applied and used as a negative control.
CLINICAL EXAMINATION AND SCORING
Skin reactions, such as erythema, oedema, or other types of skin irritation, are evaluated at 15 minutes, 1 hour, and 24 hours after patch removal. With an IIM (Mean Irritation Index, according to the amended Draize classification from 0 to 8) ranging from 0.00 to 0.32, all the three tested product can be considered suitable for sensitive skin.
15 minutes, one hour and 24 hours after Finn Chamber removal. A Finn Chamber containing a blotting paper disk soaked with demineralized water is applied and used as a negative control.
THE SKIN BIOTIC ANALYSIS
Production and Analysis Protocol
To ensure product quality, AWvi R&D begins by producing a pilot batch to assess industrial scalability and monitor product stability over time. Production then occurs in environments with optimal, controlled conditions, specifically maintaining temperature below 25°C (77°F) and humidity levels between 21-23%, to preserve the probiotics' viability and guarantee the finished product's shelf life. Furthermore, every batch produced undergoes rigorous analysis and inspection by Micro S.r.l., a specialized laboratory with external certification. This lab operates under a Quality System compliant with the UNI CEI EN ISO/IEC 17025 standard and boasts accreditation from ACCREDIA (ILAC-MRA). Additionally, it is listed in the Lombardy Region as an officially authorized laboratory for conducting analyses in accordance with food industry self-monitoring procedures (HACCP).
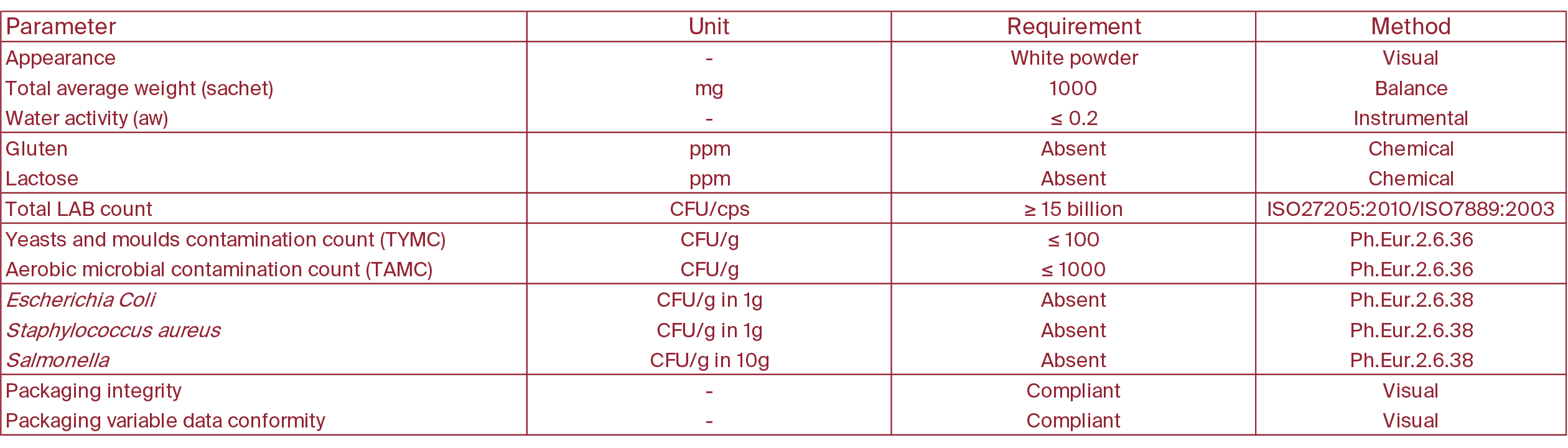
Analysis Parameters and Requirements
Analysing each production batch according to the specified parameters and requirements enables us to ensure the product's efficacy and safety, while also certifying it is free from substances that may trigger sensitivities or intolerances, such as lactose and gluten.
Your Questions About AWvi
Are AWvi products natural or organic?
AWvi has worked diligently to find the perfect balance between what's best for you and the environment, leveraging the finest offerings from both nature and science. Our products are vegan, lactose-free, and gluten-free, ensuring the safety of our solutions—free from any type of allergen—and meeting user expectations. AWvi has selected ingredients and production processes that maximize efficacy and safety, all while preserving the environment. It's crucial to note that natural does not inherently mean safe or effective. A natural extract could be harmful or contaminated, or it might prove ineffective if not processed correctly to extract the key active ingredients at the necessary concentrations to produce the desired effects. Moreover, the cultivation and extraction processes for natural components can significantly harm the environment in terms of land use, water, energy consumption, and polluting extraction processes.
If our active ingredients are of natural origin, such as ectoine and hyaluronic acid molecules, as well as our triglycerides extracted from olives, our active waters, or our probiotics, they have been either lab-grown or derived from cutting-edge extraction processes, including soft-extraction and ultra-filtration. These processes focus on precision and concentration in terms of efficacy while providing all the necessary safety guarantees.
The best example of our approach that reconciles nature and science is the use of upcycled biologically active waters derived from Mediterranean organic fruits. This transforms a functional ingredient—which can represent up to 85% of a topical solution—into an active biomimetic ingredient, with compounds that replicate the liquid composition of skin cells. In addition, our use of upcycled waters helps preserve the environment by avoiding the use of demineralized water.
What does medical grade imply?
Products incorporating medical-grade ingredients are meticulously developed and assessed by healthcare experts, utilizing potent actives. This signifies that such formulations undergo rigorous clinical evaluations to affirm their benefits, ensuring they deliver superior outcomes. The comprehensive clinical validation, coupled with the advanced expertise of the professionals involved, assures users of the products' exceptional quality and safety.
For The Skin Biotic, both the manufacturing process and the packaging adhere to stringent protocols inspired from the pharmaceutical industry, guaranteeing the safety, efficacy and longevity of the product.
Is long-term use of AWvi solutions recommended and beneficial?
AWvi formulas are composed of biomimetic ingredients, developed to mirror the body's natural physiological processes, and work in harmony with the individual's unique skin biology to promote rebalancing and regeneration. By leveraging the body’s intrinsic mechanisms with a very precise dosage of ingredients, AWvi solutions are expertly designed to provide benefits both at short term and on the long run.
To draw a parallel with a daily used product, consider mineral waters. Some waters may have a composition that is not entirely balanced, beneficial in the short term in the case of certain deficiencies in some specific minerals, but not recommended in the long term, making preferable to rotate brands to avoid an excess of certain minerals that the body is not able to expel. If such a principle may be applicable in the cosmeceutical and nutraceutical fields, AWvi's carefully calibrated formulations are distinct, having been specifically designed to consistently support the skin's health, remaining highly efficient and safe for ongoing use, thus negating the need for such rotation.
In essence, AWvi embraces the principle of 'just right'—meticulously blending the best of nature and science to create solutions that are as safe for long-term use as they are effective, echoing the perfect balance found in nature's own systems. The longer you integrate AWvi solutions into your daily ritual, the more significant the benefits become.
