We have substantiated our efficacy claims through clinical studies, performed by a third party (Complife Group1).
1 Efficacy clinical study performed by Complife Group, a multi-certified third party providing microbiological and chemical-physical testing. All the study procedures are carried out in compliance with the ethical principles for the medical research (Ethical Principles for Medical Research Involving Human Subjects, adopted by the 18th WMA General Assembly Helsinki, Finland, June 1964 and amendments).
AIM OF THE STUDY
The study has been designed — in the current framework proposed by Commission Regulation (EU) No 655/2013 — to assess the all-encompassing effectiveness of The Skin Method's full product range in addressing multiple benefits related to skin aging and skin health. In particular, the study aims to explore the synergistic impact of using The Skin Biotic nutraceutical in combination with the three cosmeceutical (topical) products—The Gentle Cleanser, The Power Serum, The Active Cream.
SUBJECT
→ Multicentric, double-blind, randomized, placebo-controlled study.
→ 96 healthy subjects, divided into 4 groups of 24 subjects each, enrolled by a board-certified dermatologist, according to specific inclusion and exclusion criteria.
- Group 1: 24 subjects are treated with The Skin Biotic + Placebo topical solutions
- Group 2: 24 subjects are treated with Placebo supplement + Placebo topical solutions
- Group 3: 24 subjects are treated with The Skin Biotic + AWvi topical solutions
- Group 4: 24 subjects are treated with Placebo supplement + AWvi topical solutions
→ Main inclusion criteria: Age range: 45 to 65 years, with an average age of 55 years; Including sensitive skin; Skin ageing from mild to moderate.
→ 8 weeks study duration; Assessment of the product effects after 4- and 8-week use.
RESULTS
Evidence-backed outcomes
Transformative results in 8 weeks
MEASURED PARAMETERS
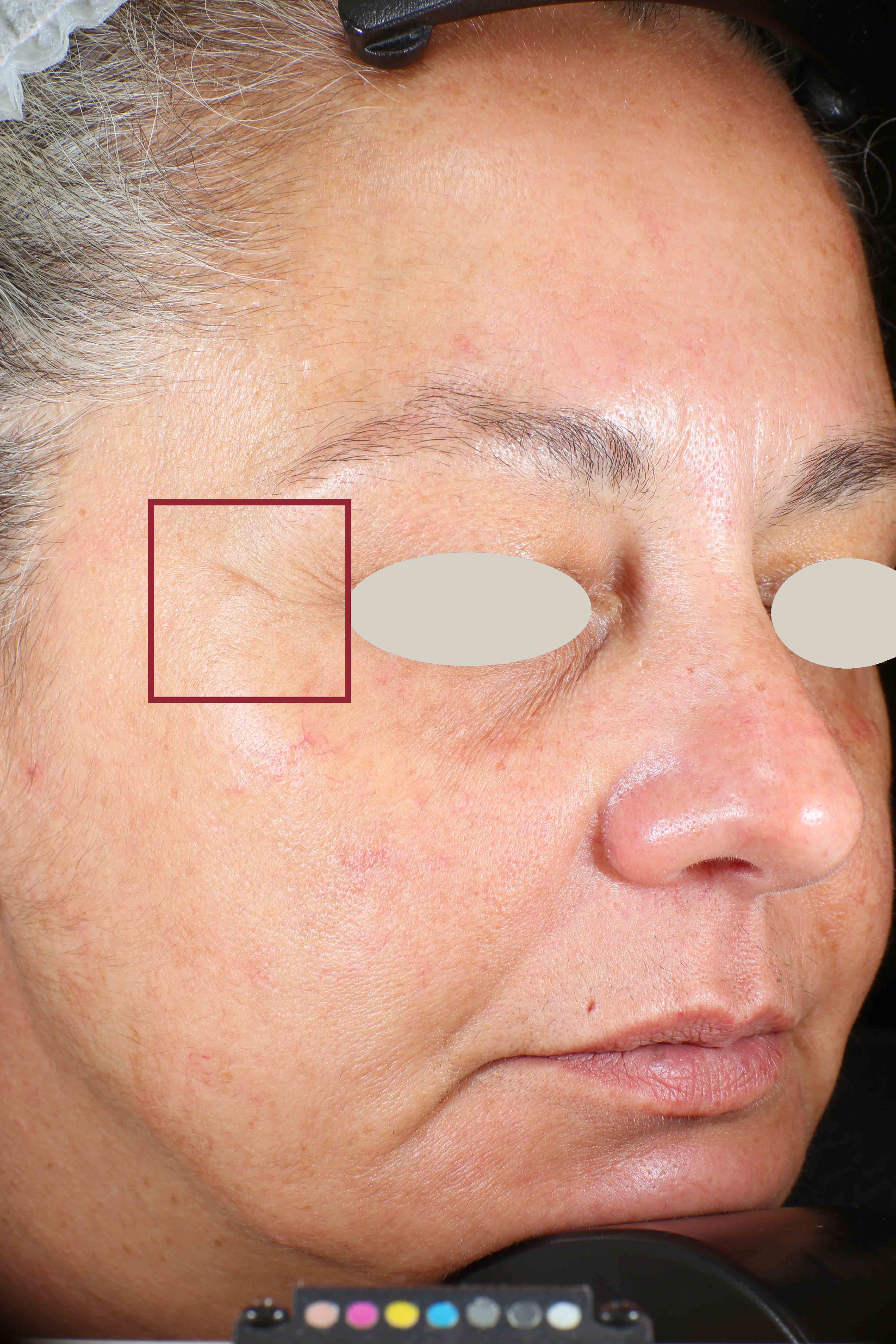
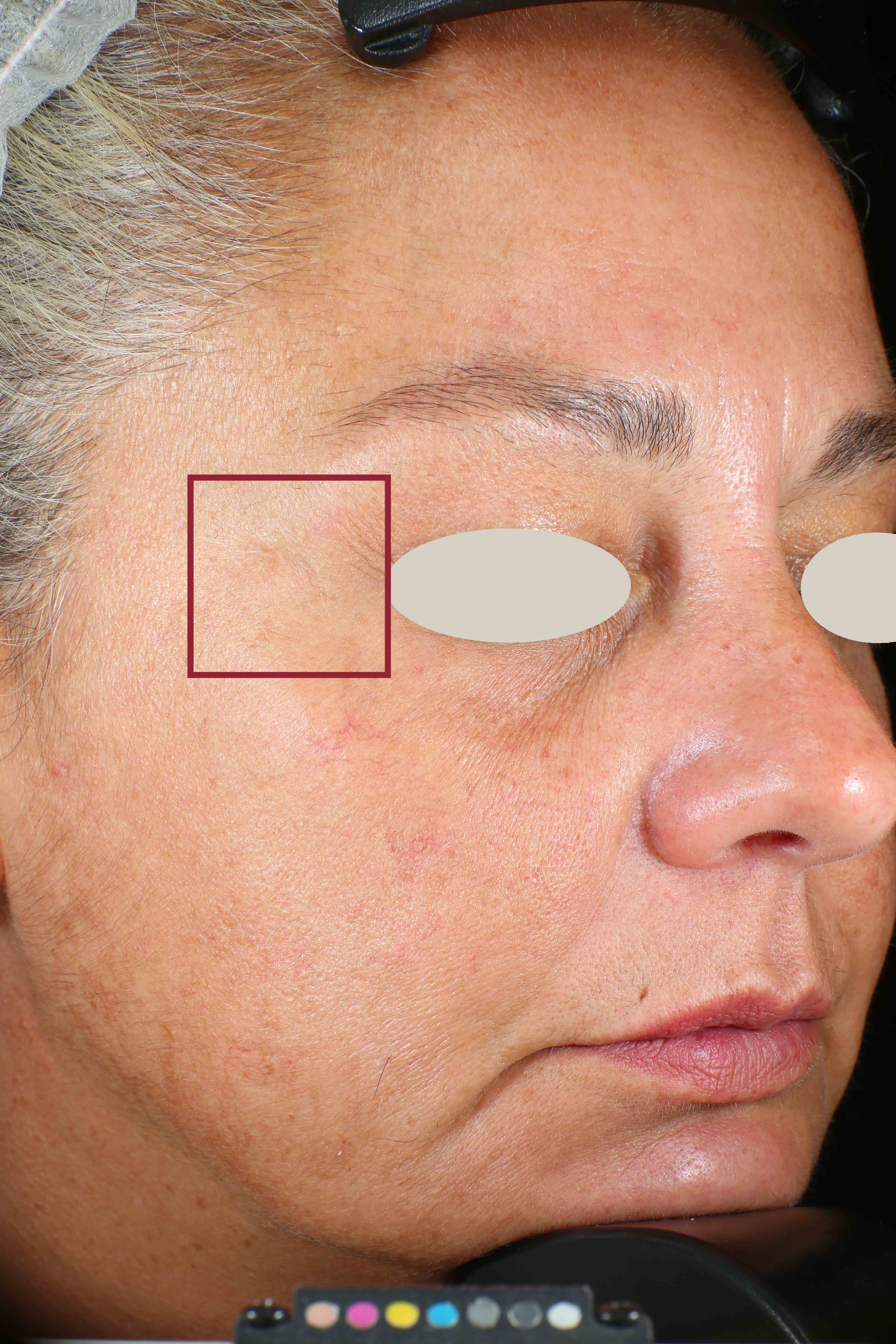
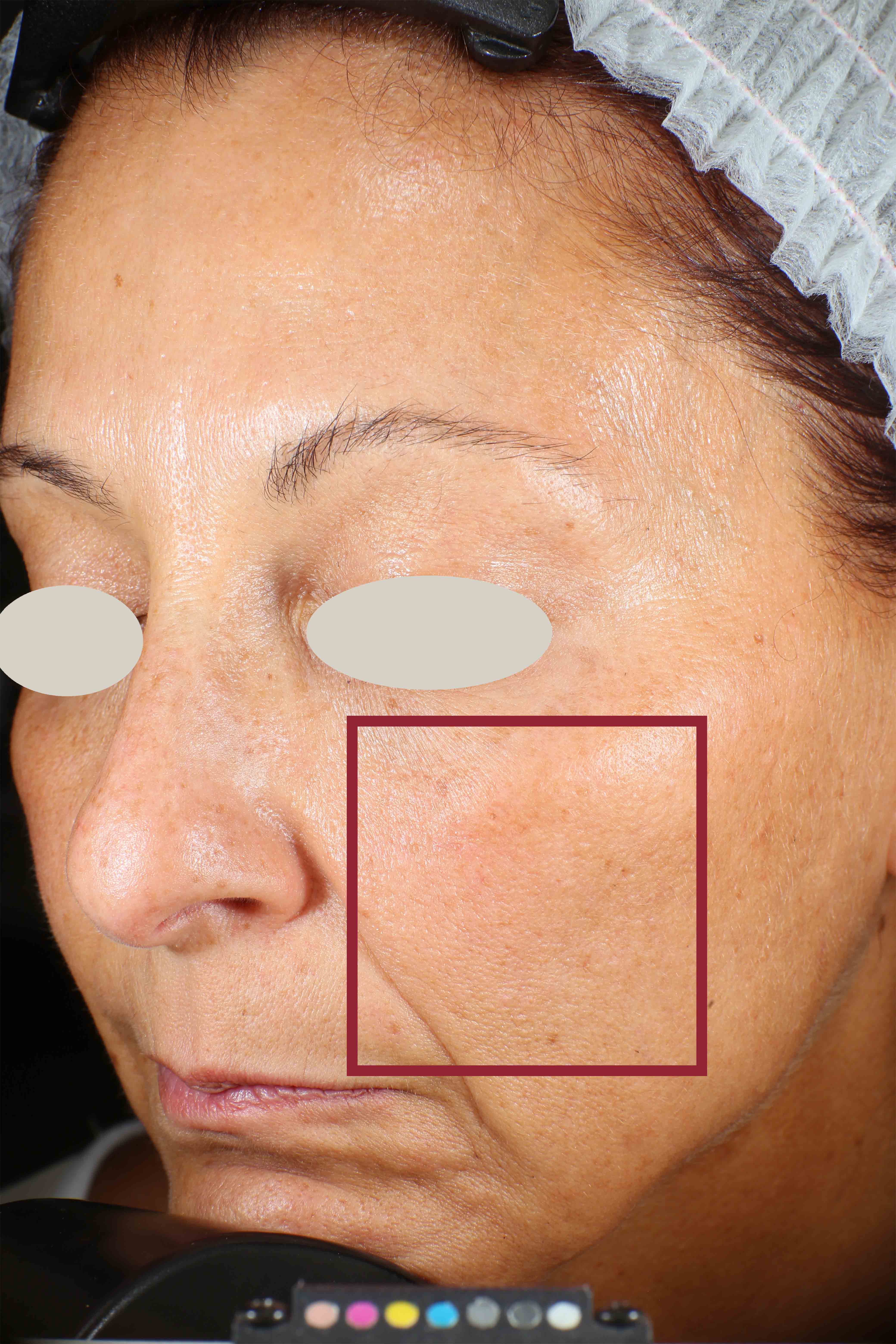
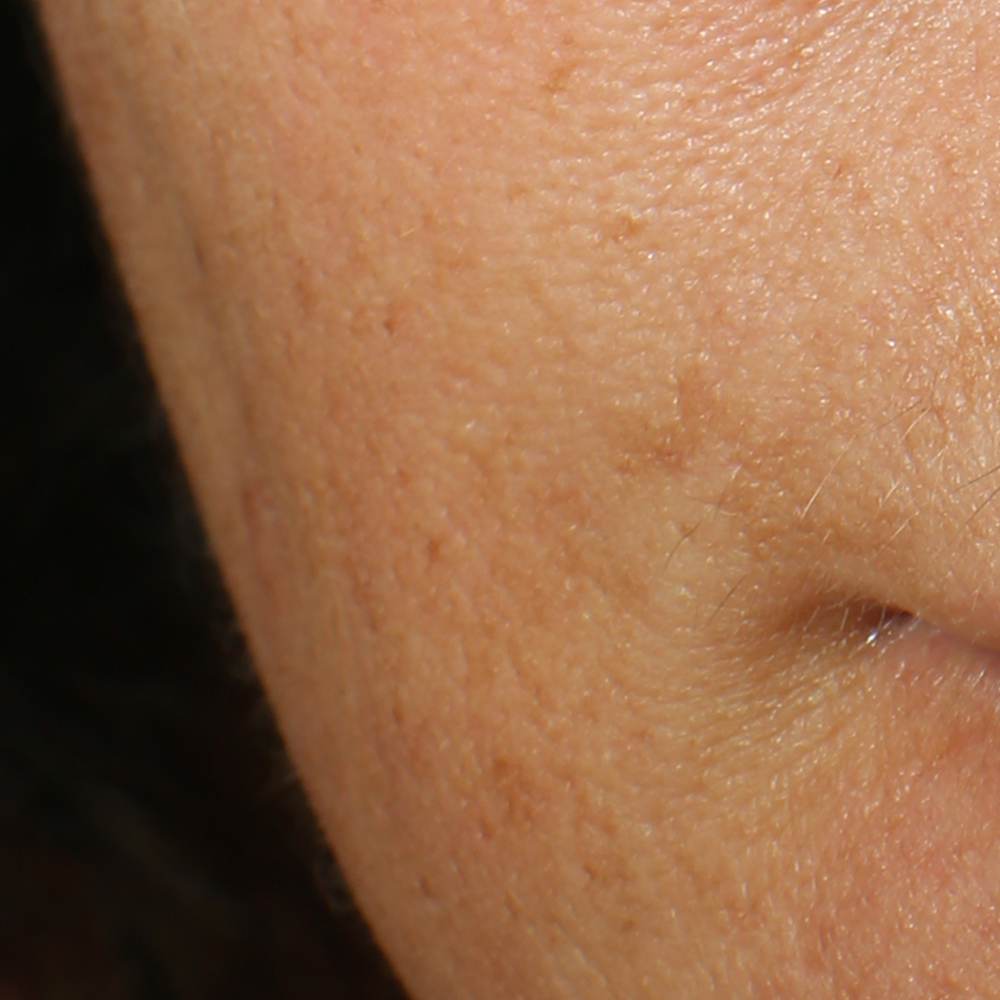
Wrinkles and Fine Lines reduction
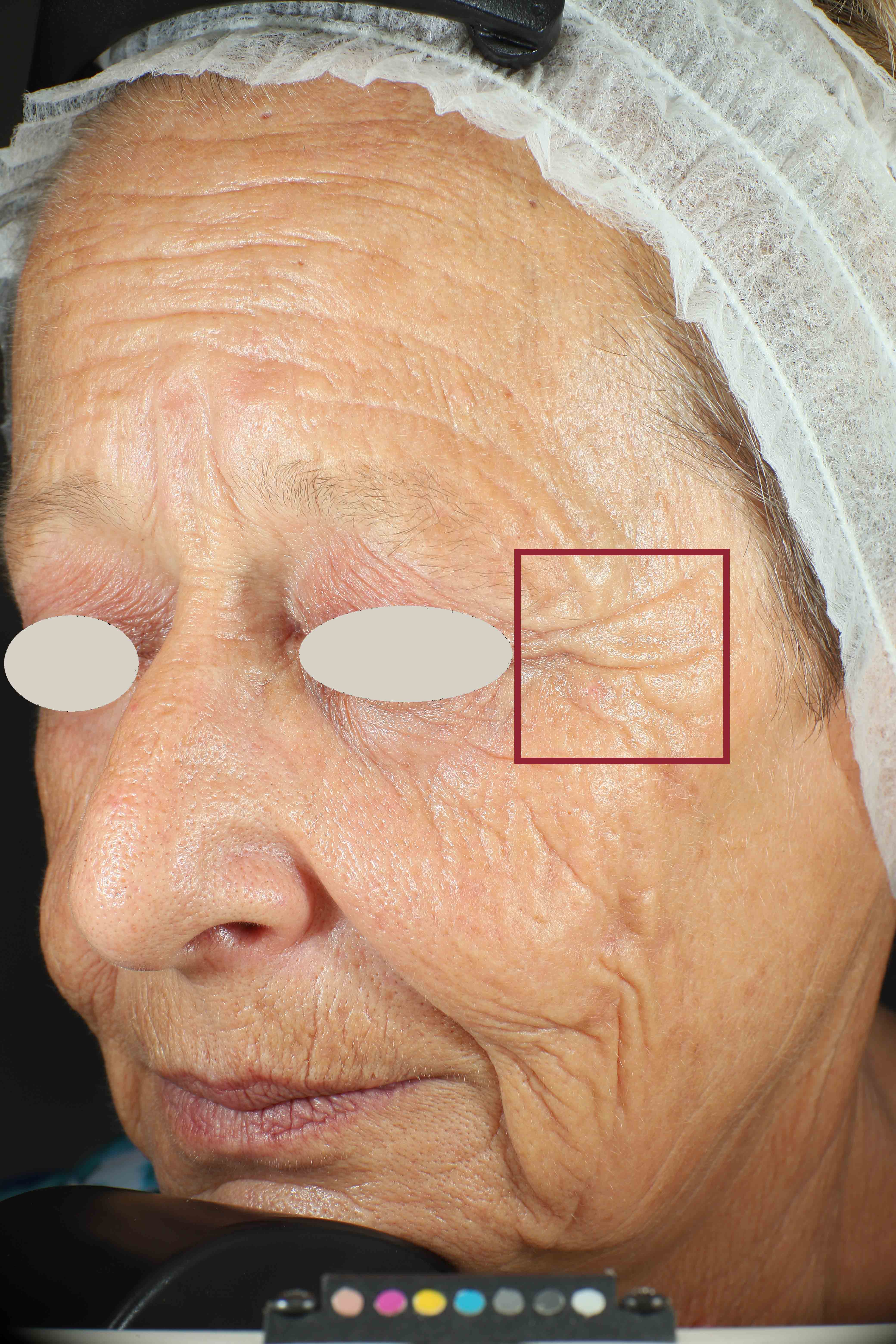
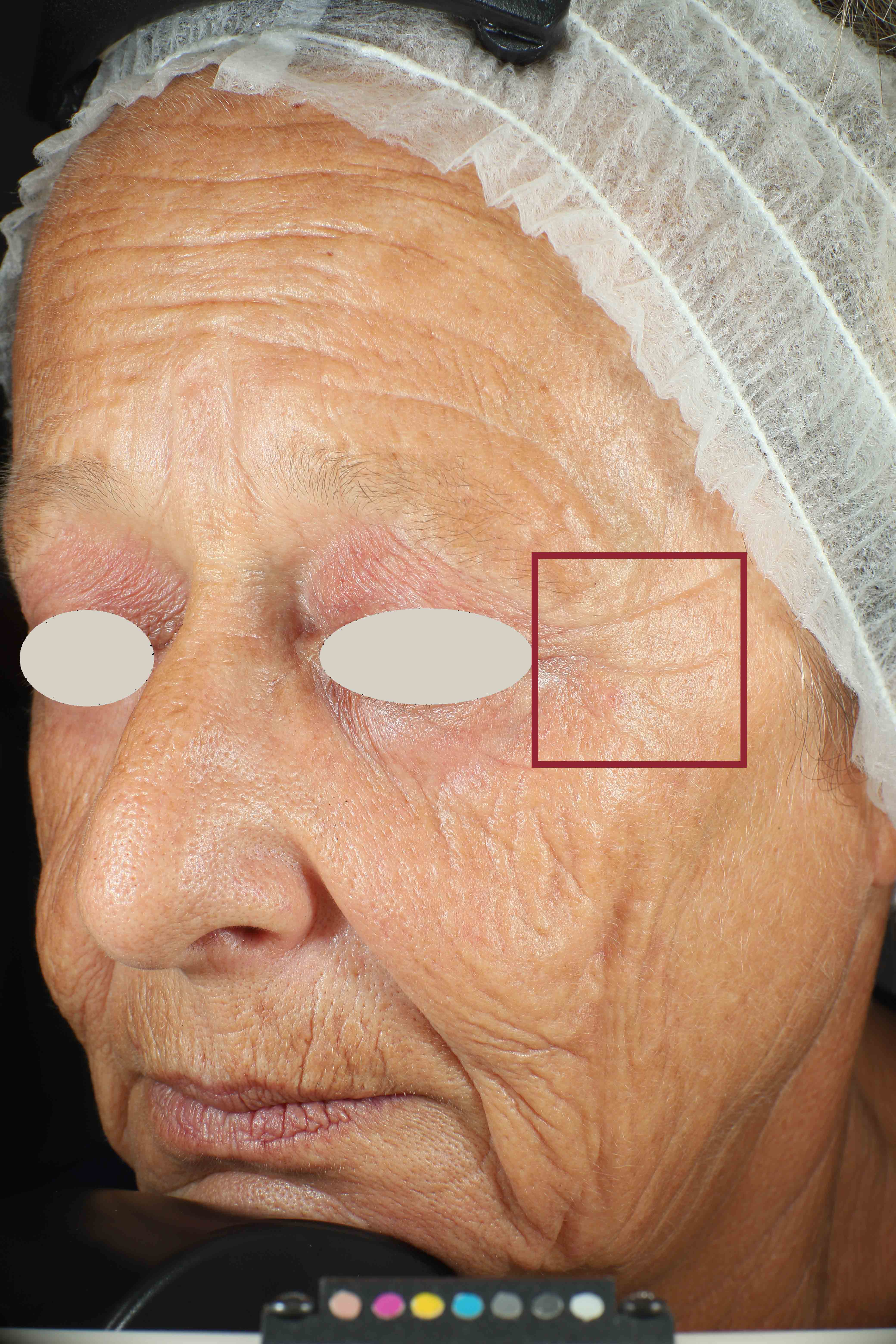
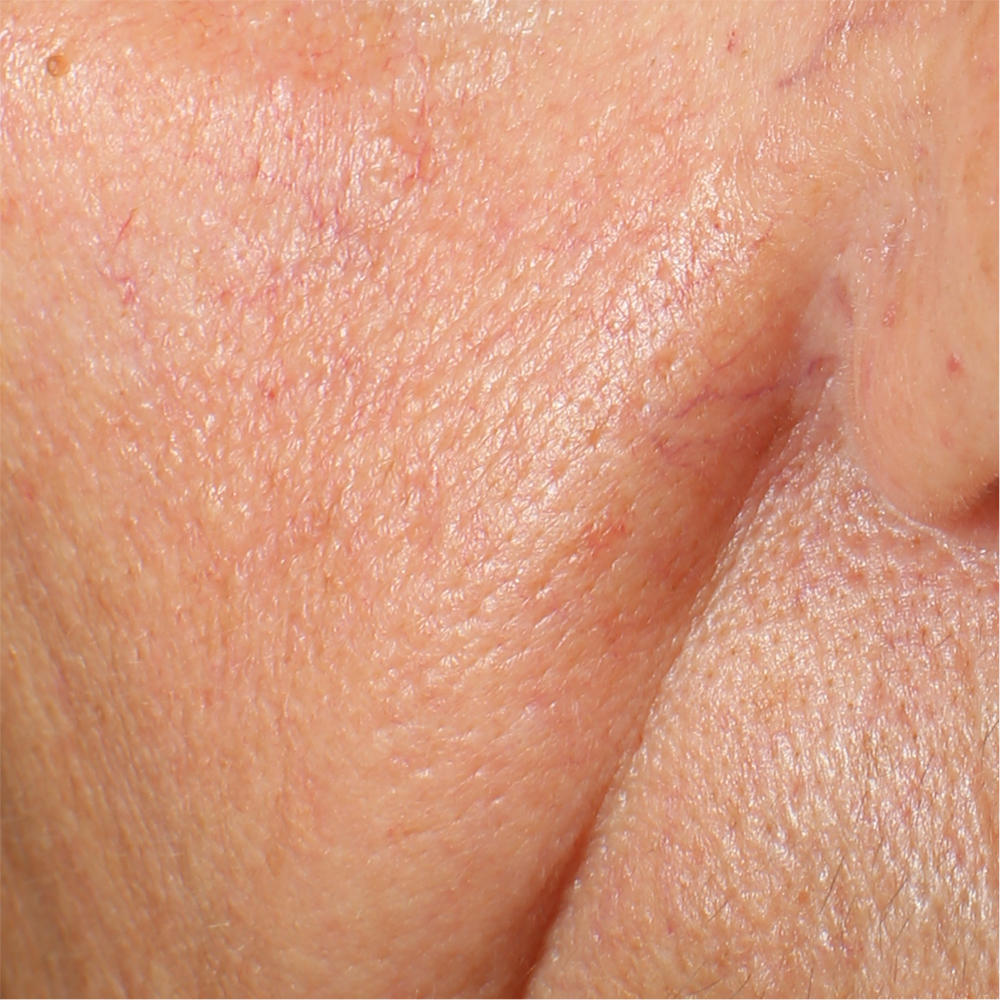
Skin surface (i.e. wrinkle depth, volume, skin roughness, etc.) is quantitatively assessed by Primos CR SF (Canfield Scientific Europe, BV, Utrecht, Netherlands). Primos CR SF is a non-contact in vivo skin measurement device based on structured light projection. In conjunction with a comprehensive 3-D measurement and evaluation software, the sensor allows to evaluate skin surface properties.
In this study:
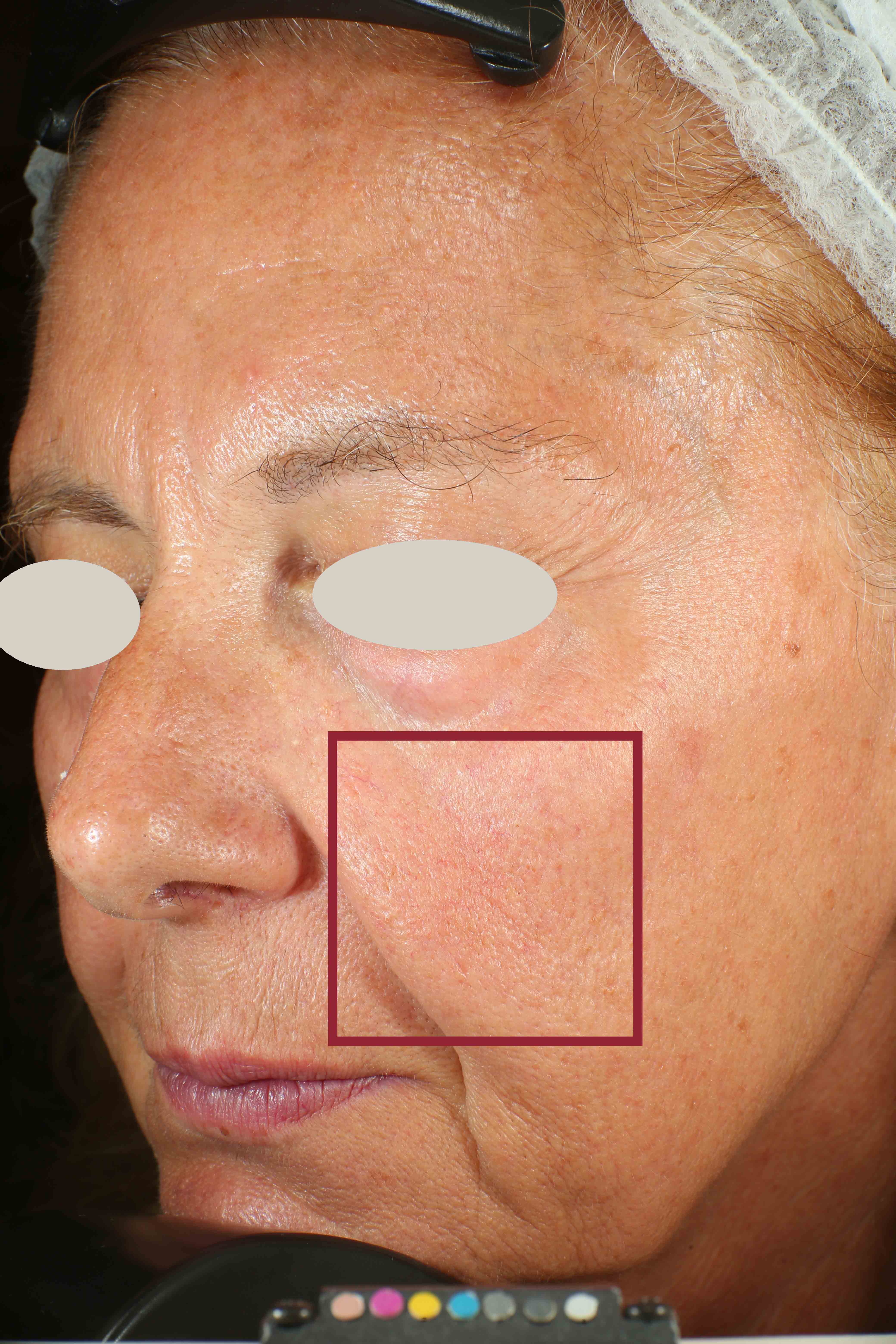
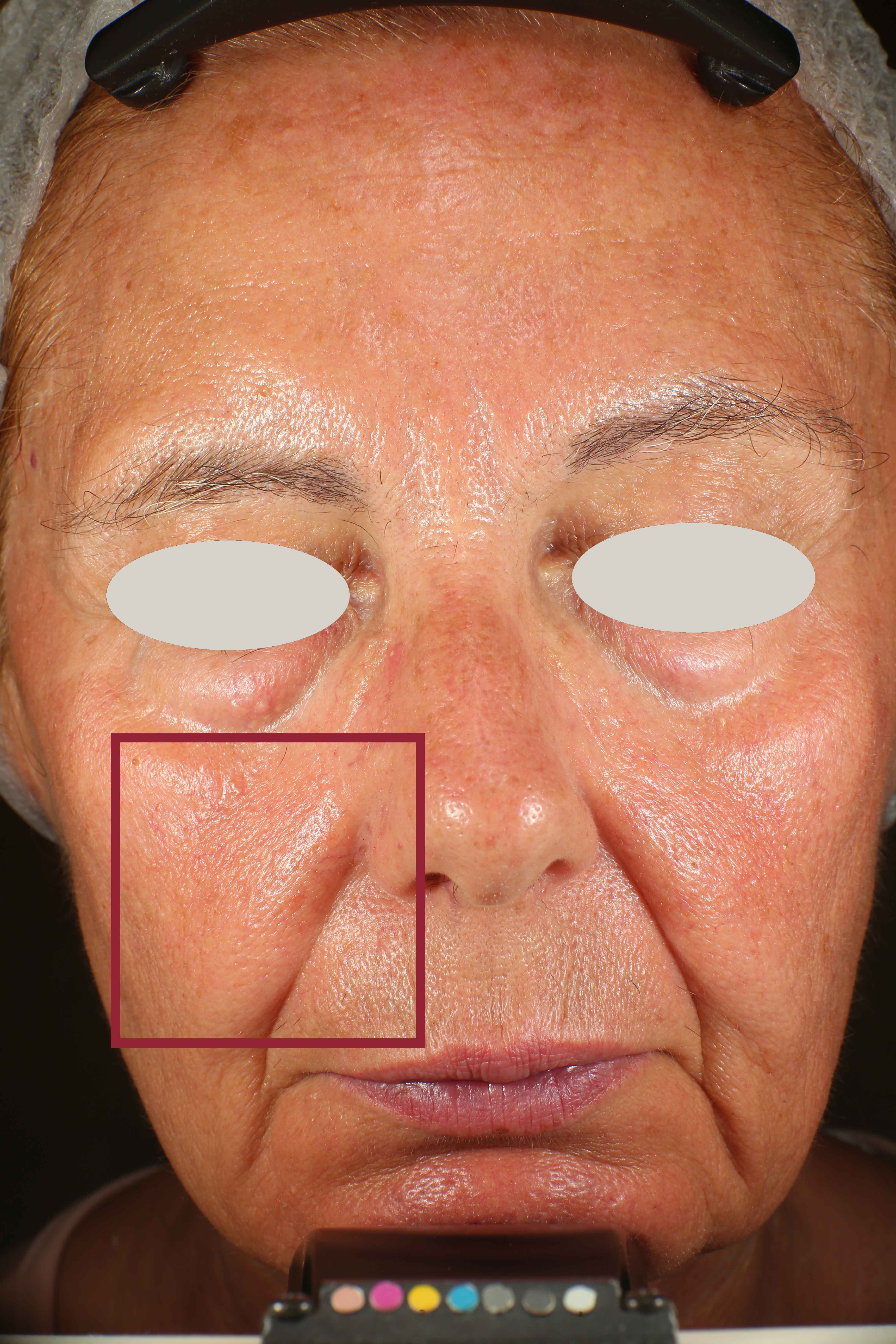
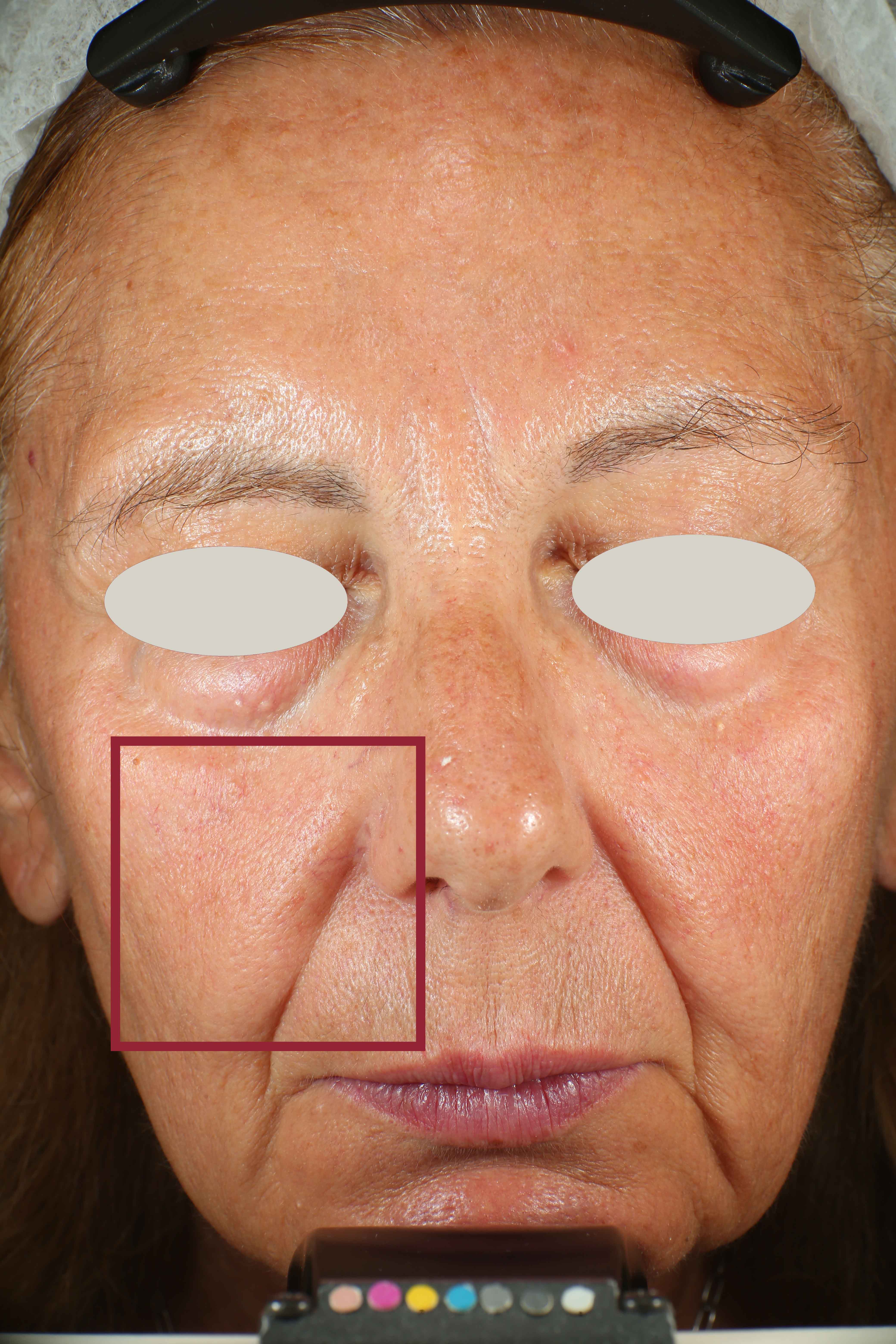
- Sa parameter (related to skin smoothness) is measured in the crow’s feet area at T0/T4/T8
- Crow’s feet wrinkle depth is measured at T0/T1h/T4/T8.
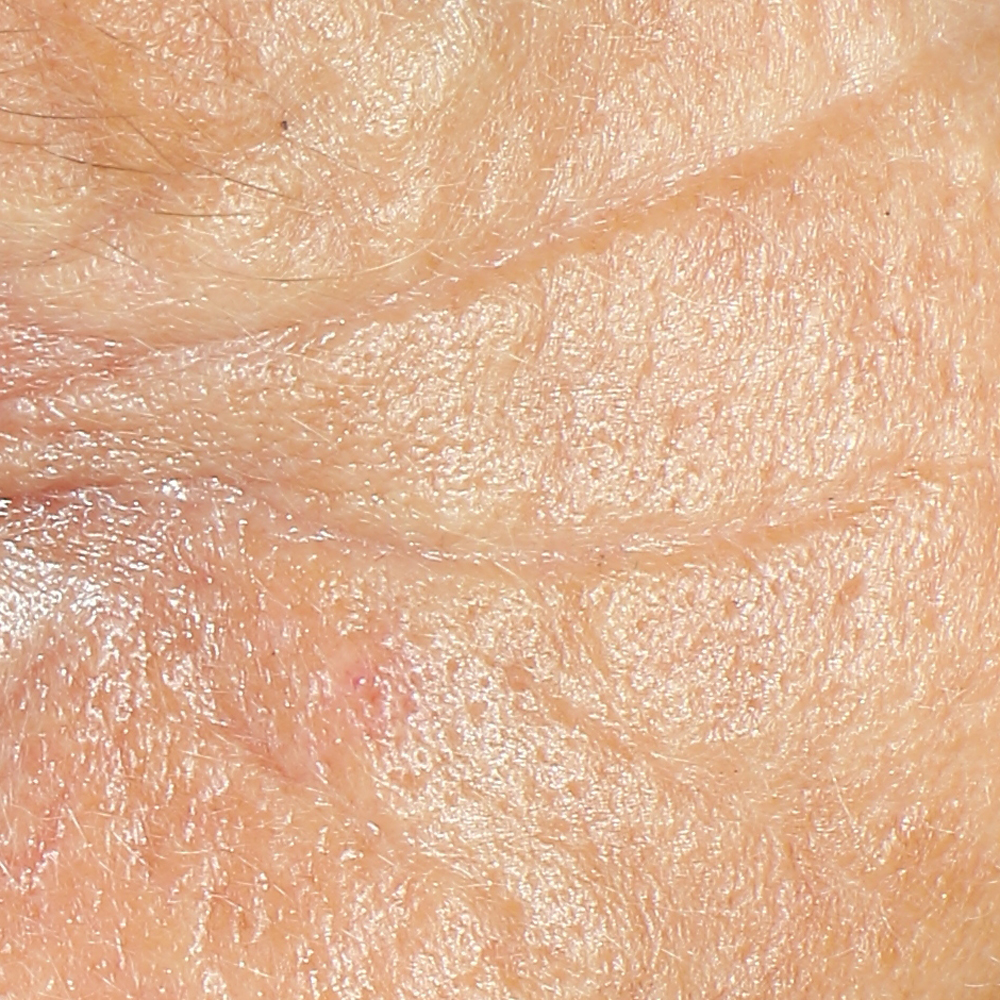
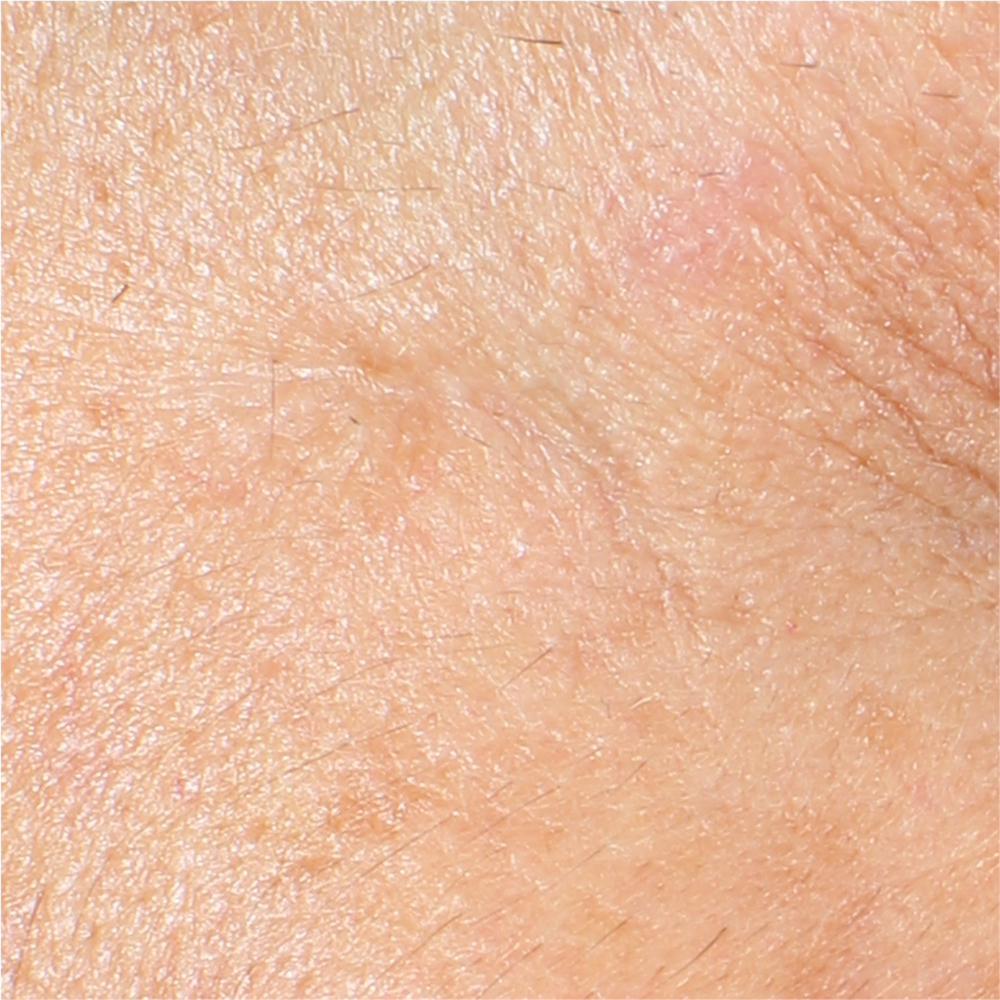
Skin profilometry by means of Primos CR SF analysis:
a)
b)
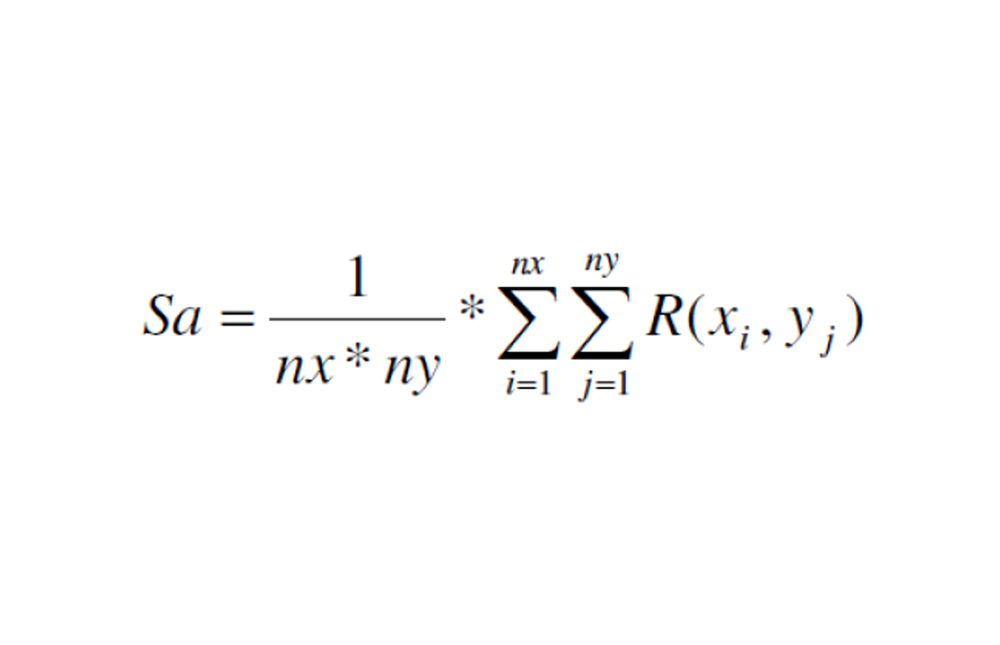
c)
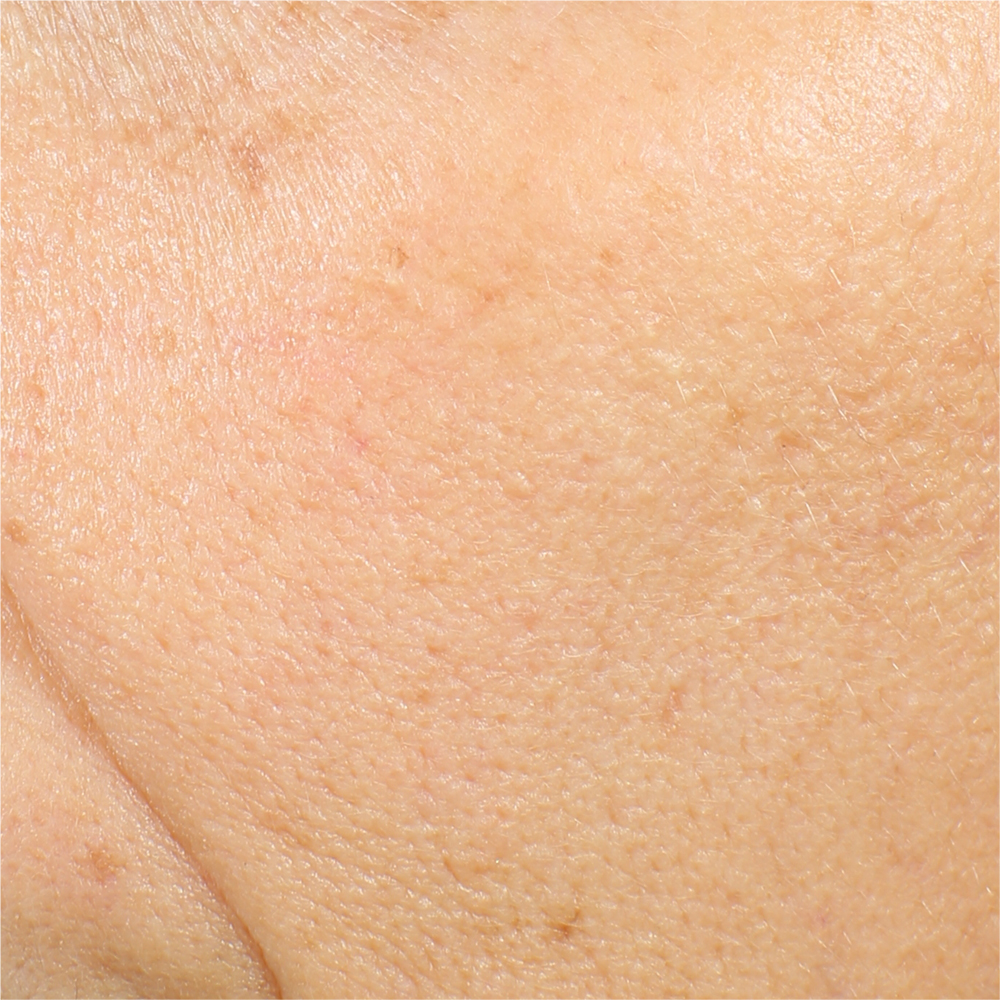
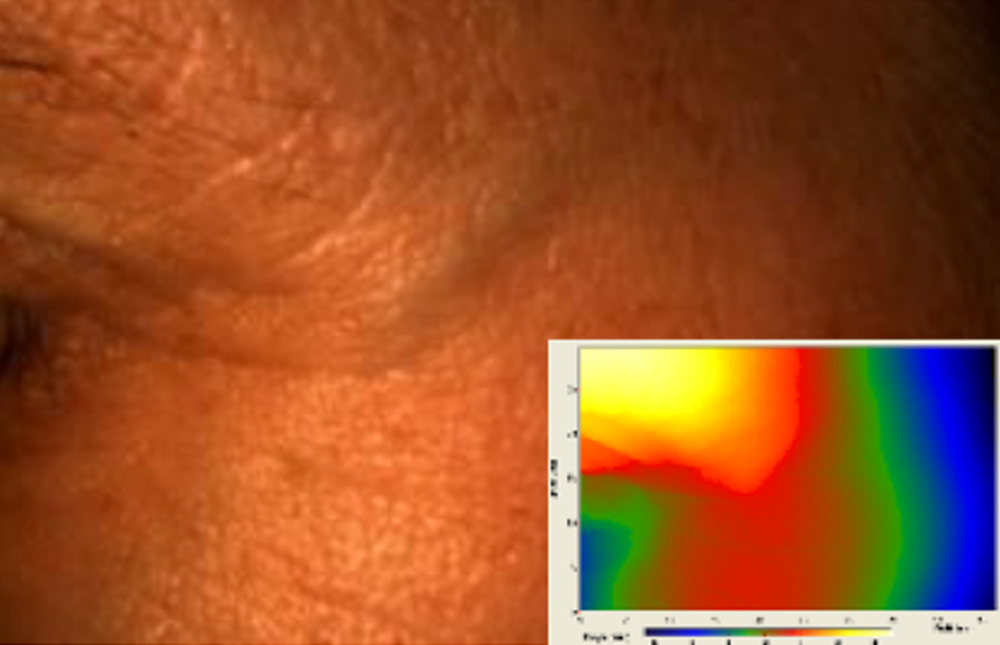
a - The technique.
Primos CR SF (small field) is a 3D scanner that create a point cloud (set of vertices in a three-dimensional coordinate system) of geometric samples on the surface of the subject. These points are then used to extrapolate the shape of the subject (a process called reconstruction). Like cameras, 3D-scanner has a cone-like field of view, and like cameras, they can only collect information about surfaces that are not obscured. While a camera collects color information about surfaces within its field of view, 3D scanners collect distance information about surfaces within its field of view. The “picture” produced by a 3D scanner describes the distance to a surface at each point in the picture (see the image in the insert).
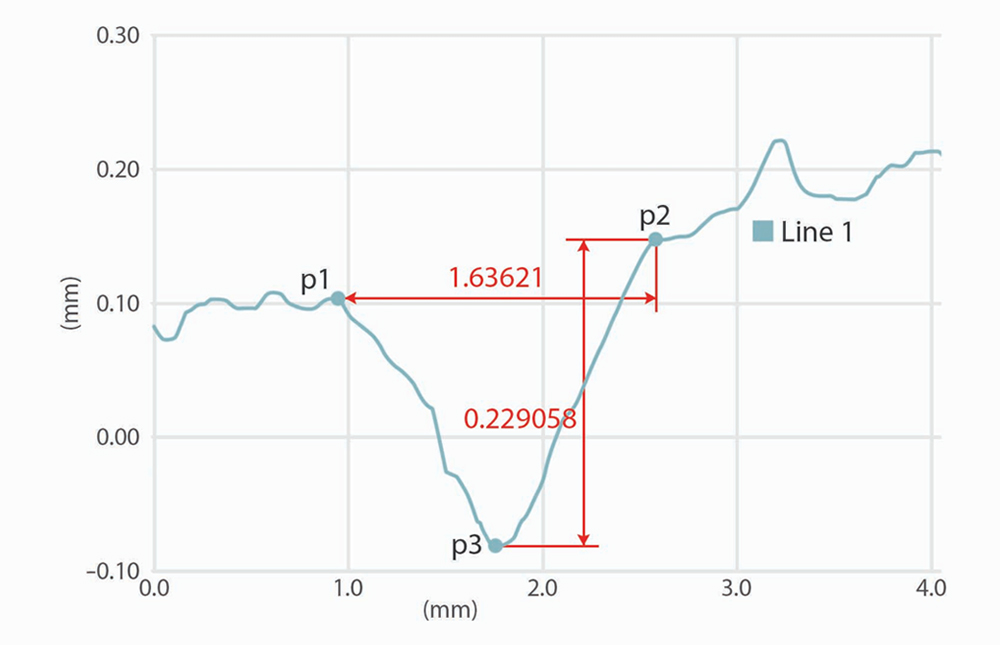
b - Calculation of wrinkle depth.
It is calculated the height of wrinkles in the sampling lengths: this calculation is done on the sectional picture (wrinkle depth vs. section).
c - Calculation of skin smoothness (Sa parameter).
The surface roughness is calculated through the arithmetic roughness (Sa parameter). Sa is the arithmetic mean of the surface roughness and it is a vertical parameter. This means it describes the roughness in vertical direction. The parameter is used to evaluate the surface smoothness.
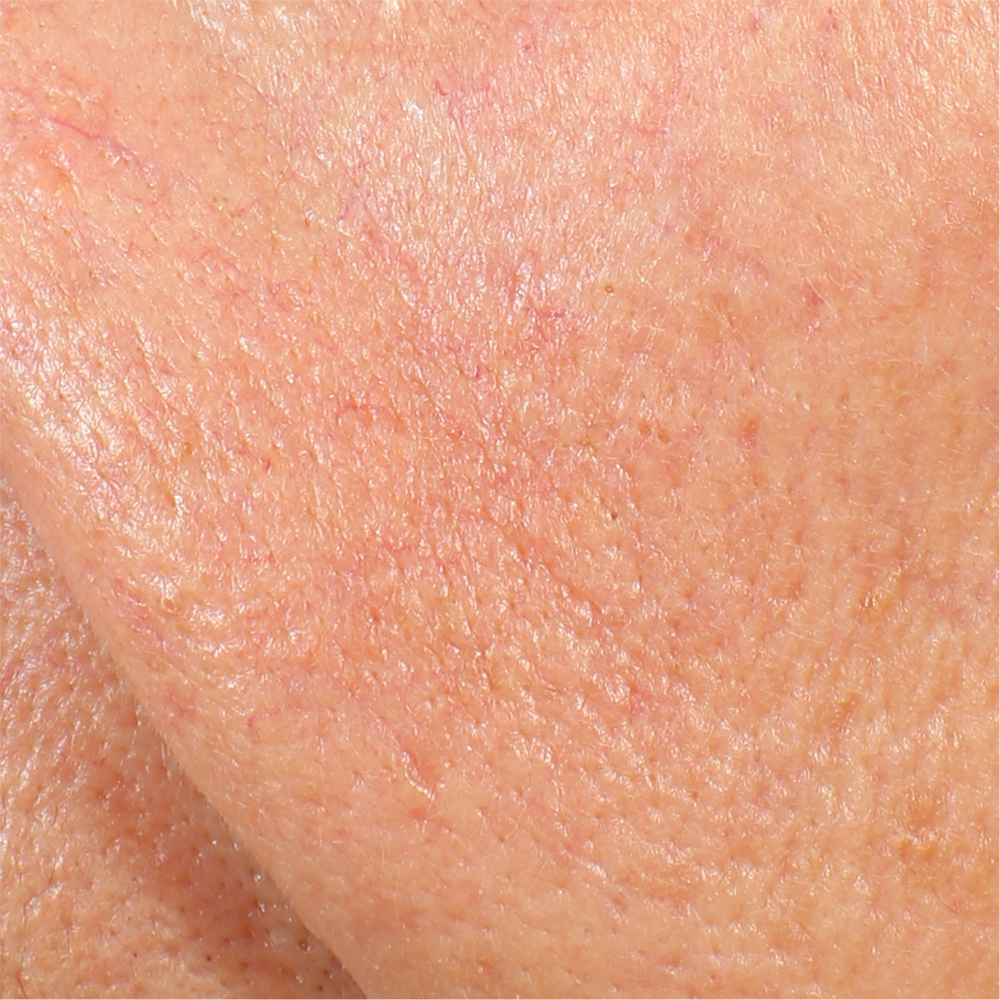
Skin radiance improvement
Skin radiance (or skin brightness) is the ability of the skin to reflect the light and it is measured using the gloss parameter (taken using the spectrophotometer/colorimeter CM-700D (Konica-Minolta). The instrument emits diffuse light that reaches the skin through an opening located at the extreme of the lighting sphere. A sensor located at 8° compared to the vertical axis of the opening detects then the reflected light and calculates a parameter known as ‘gloss’. The gloss value is used in the management of the brilliance of the colour. Skin radiance is measured at T0/T1h/T4/T8.

Skin moisturisation improvement
Skin moisturisation (and hydration) is evaluated by means of Corneometer® measurement. This measurement is based on the completely different dielectric constant of water (81) and other substances (mostly < 7). The measuring capacitor shows changes of capacitance according to the moisture content of the skin. A metallic lamina separates the metallic tracks (gold) in the probe head from the skin in order to prevent current conduction in the measured area. An electric field between the tracks with alternating attraction develops. One track builds up a surplus of electrons (minus charge) the other a lack of electrons (plus charge). The scatterfield penetrates the very first layer of the skin (10-20 μm) during the measurement and the capacitance is determined.
Skin moisturisation is measured at T0/T1h/T4/T8.
Skin elasticity and firmness improvement
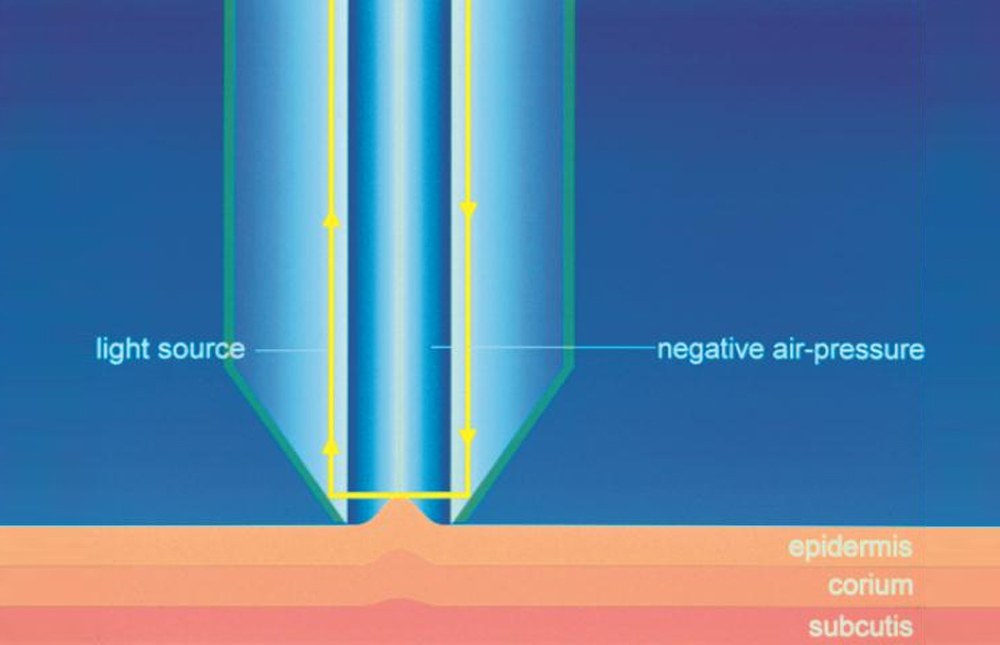
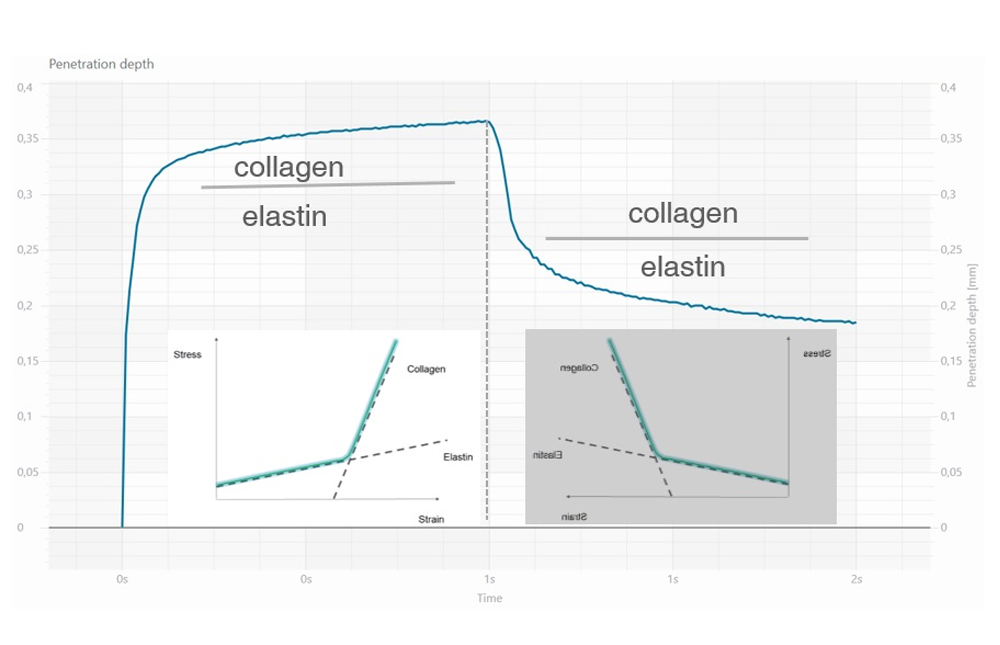
Skin elasticity measurement is based on the suction/elongation method and the subsequent release of the skin inside the opening of the instrument (Cutometer®MPA 580, Courage+Khazaka, electronic GmbH). During the suction/elongation phase the instrument generates, in fact, a constant negative pressure (450 mbar) able to aspirate the skin inside the measurement probe. The suction phase is followed by the release phase, in which the pressure inside the probe is switched to 0 mbar allowing the skin recovery after the elongation phase. An optical measurement system evaluates the depth of the skin inside the probe in the two phases of the measurement, the obtained data are then elaborated and showed graphically and numerically in order to calculate the viscoelastic properties of the skin.
In this study, the following parameter are measured:
- R2 parameter (gross elasticity or overall elasticity): it is the ratio between the residual deformation and the maximum elongation of the skin (Ua/Uf) and it indicates the ability of the skin to return to its original state of recovery after a stressing event. Closer the value is to 1, more elastic is the skin.
- R0 parameter (skin distensibility): it is the first max amplitude of the curve (Uf) and it represents the passive behavior of the skin to a force (i.e. gravity). A reduction of R0 parameter indicates an improvement of the skin ability to oppose to the deformation imposed by the probe during the suction phase, than can be expressed as an improvement of skin firmness.
Skin elasticity and firmness is measured at T0/T4/T8.
Skin barrier function improvement
Skin barrier function is measured at T0/T4/T8 using a Tewameter® TM 300 (Courage+Khazaka, electronic GmbH). The following equation which represents the Diffusion law (discovered by Adolf Fick in 1855) is the basis for the measurement:
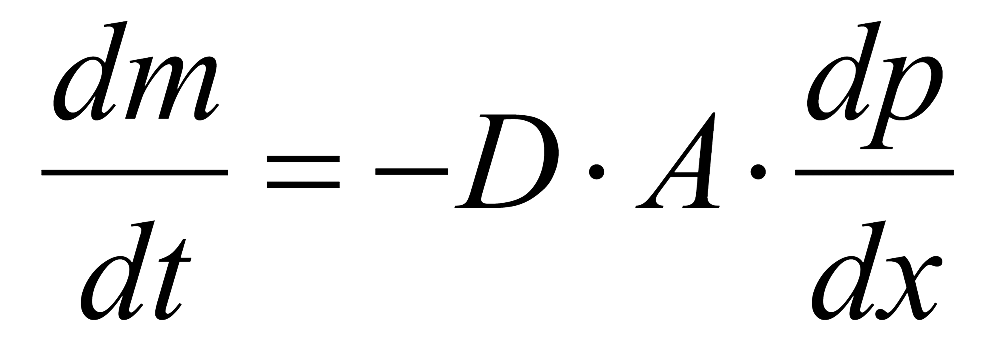
Where:
A=surface in m² / water transported (in g) - / time (h) - / diffusion constant (=0.0877 g/mhmm Hg) / vapor pressure of the atmosphere (mm Hg) - / distance from skin surface to point of measurement (m)
The diffusion flow dm/dt indicates the mass per cm² which is transported in a specific period of time. It is proportional to the area A and the change of concentration per distance (dp/dx). D is the diffusion coefficient of water vapor in the air. This law is only valid within a homogenous diffusion zone, which is approximately formed by a hollow cylinder. The resulting density gradient is measured indirectly by two pairs of sensors (temperature and relative humidity) and is analyzed by a microprocessor. The measuring head of the probe is a narrow hollow cylinder (10 mm diameter and 20 mm height), in order to minimize influences of air turbulence inside the probe.
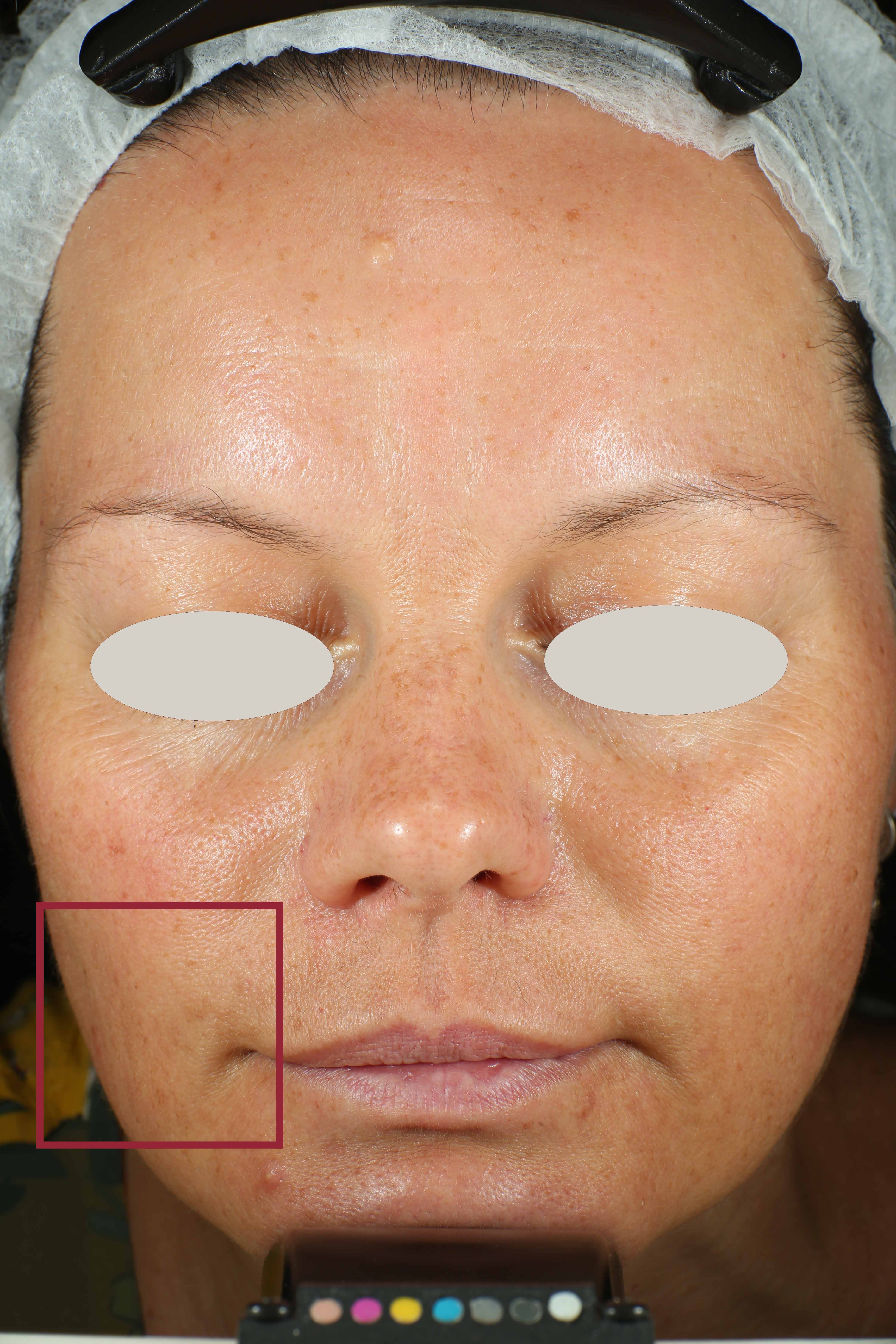
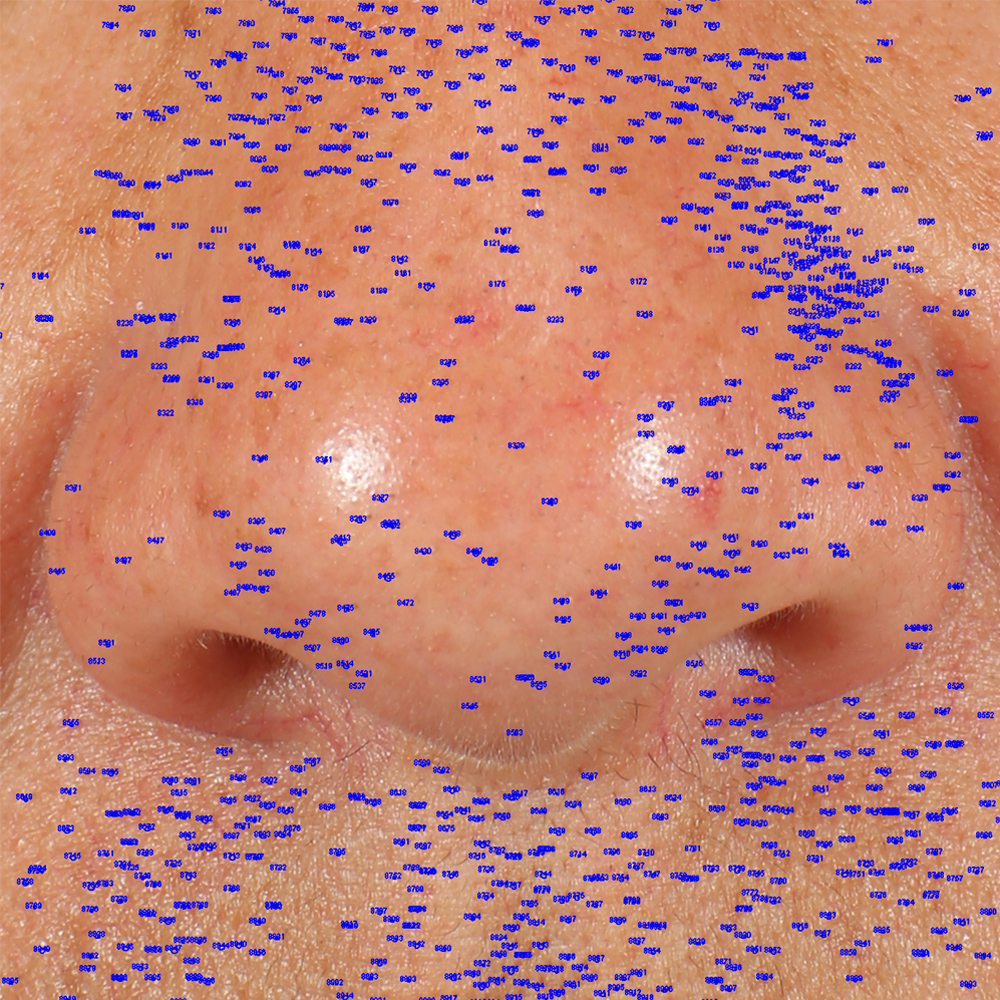
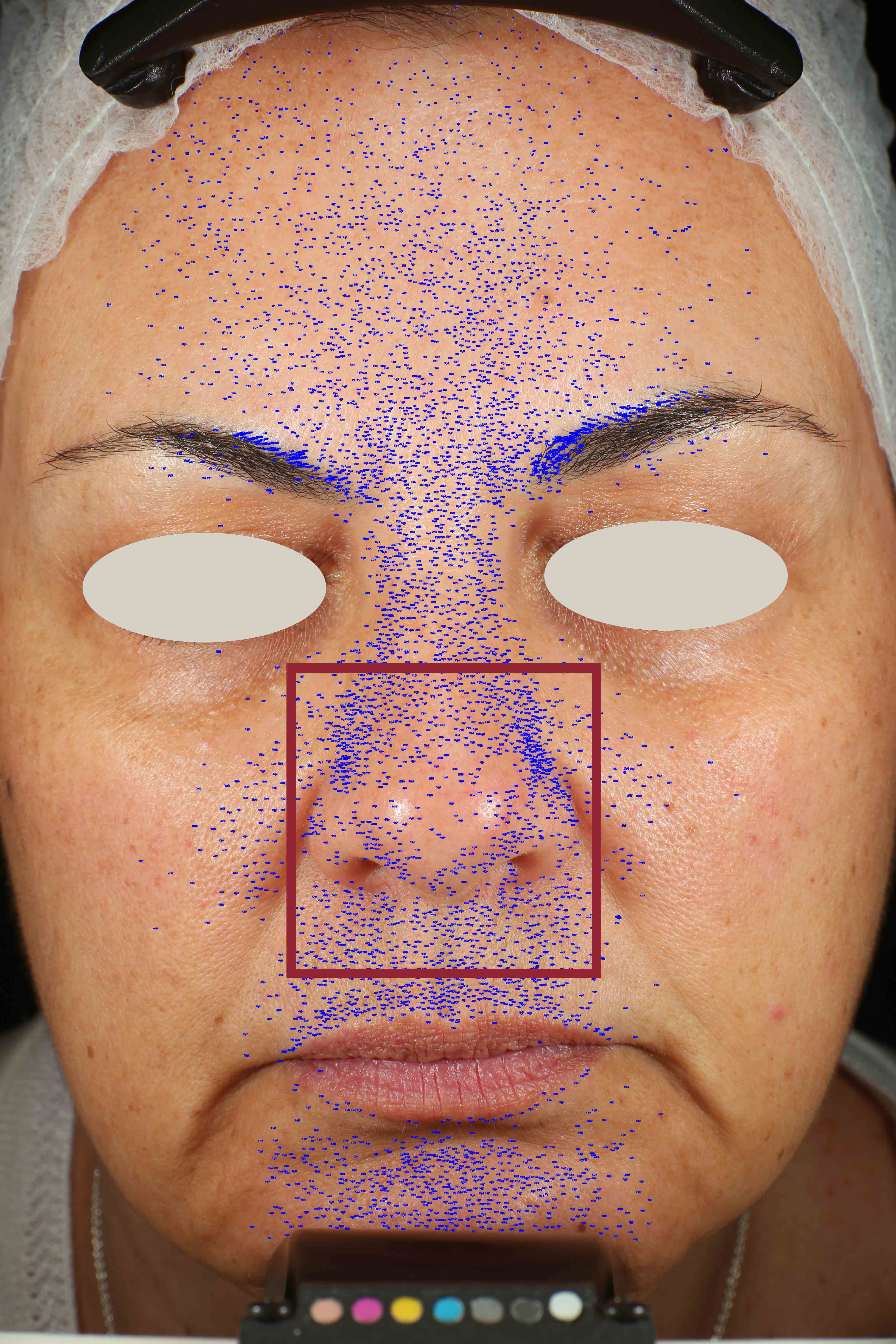
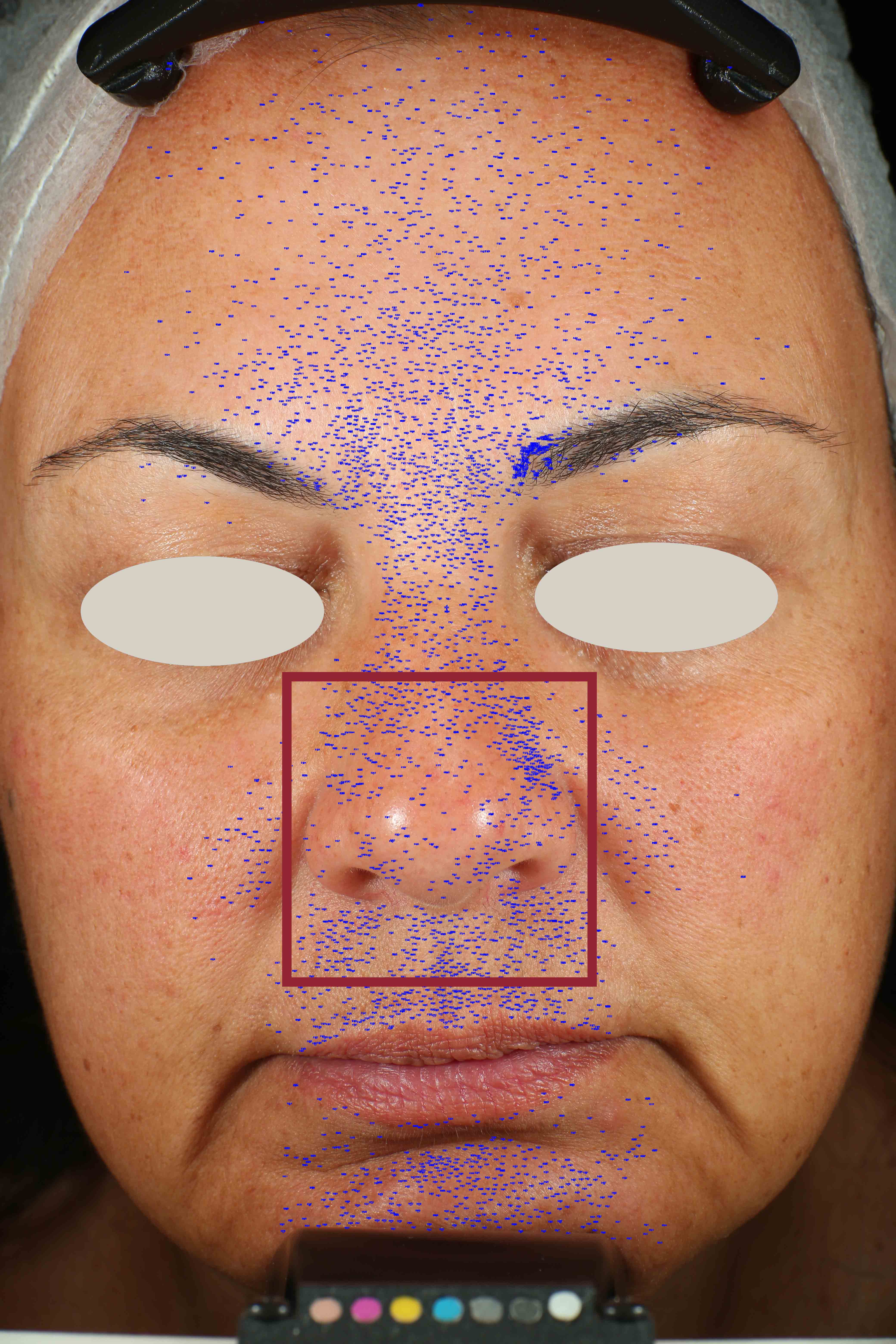
Skin impurities removal
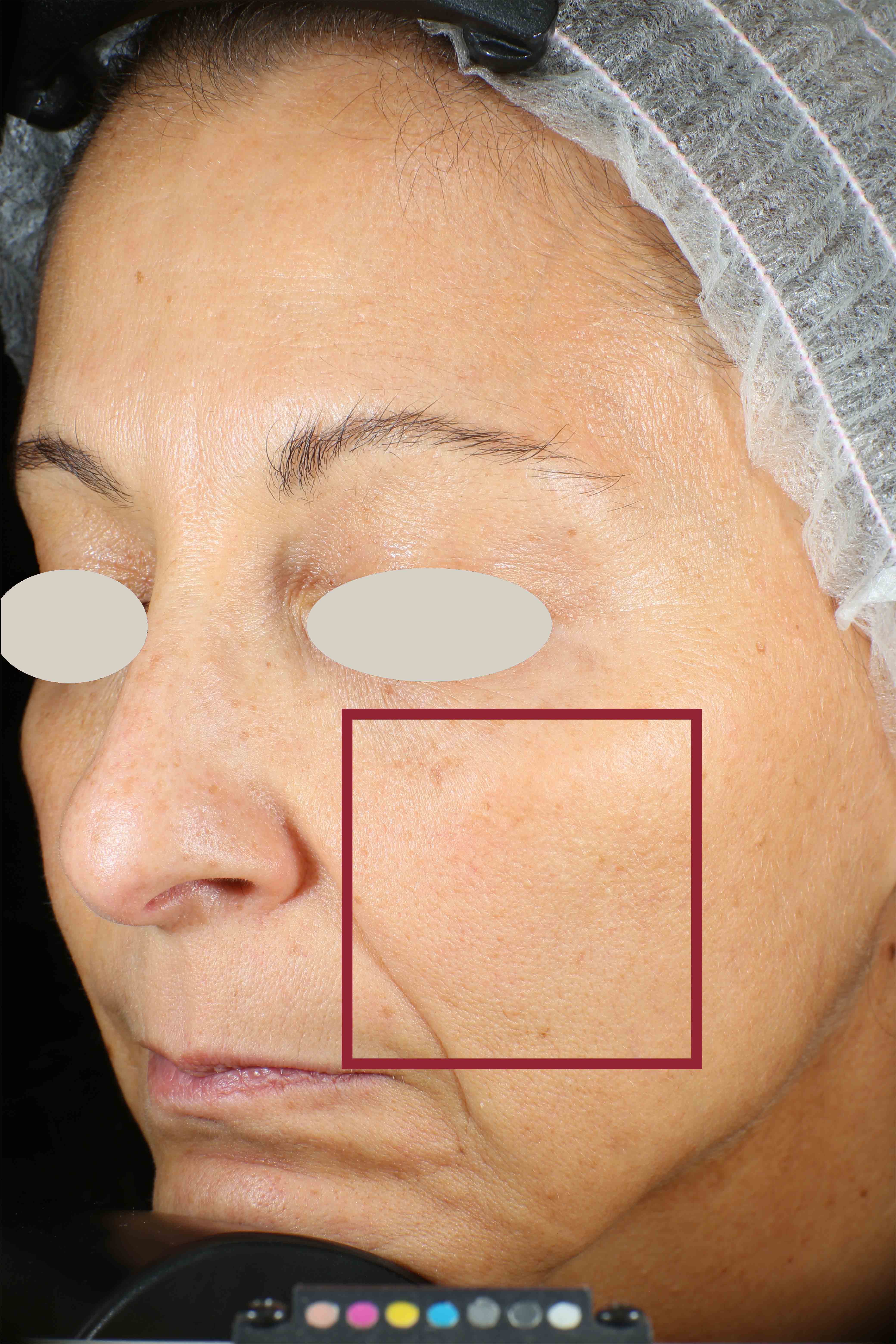
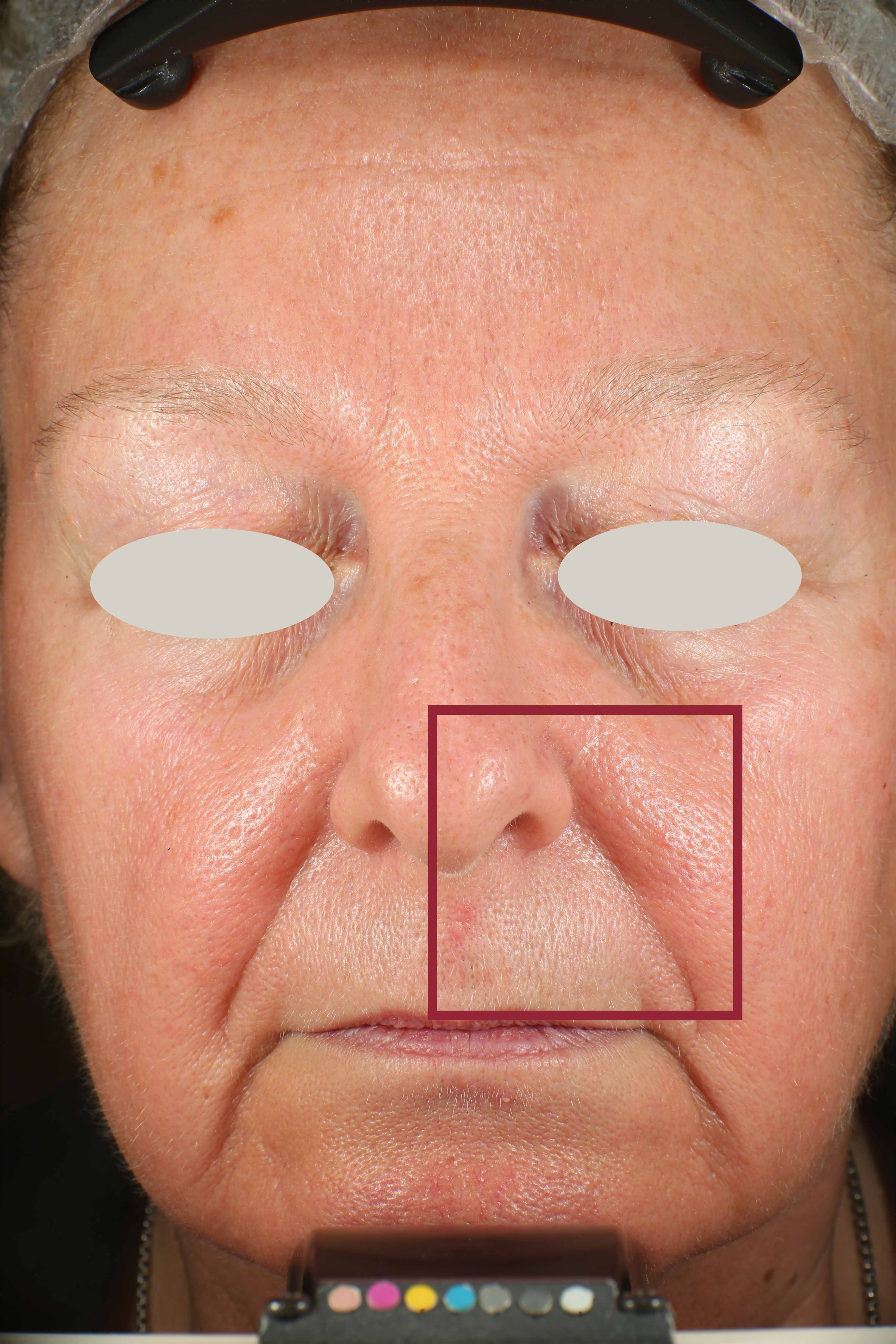
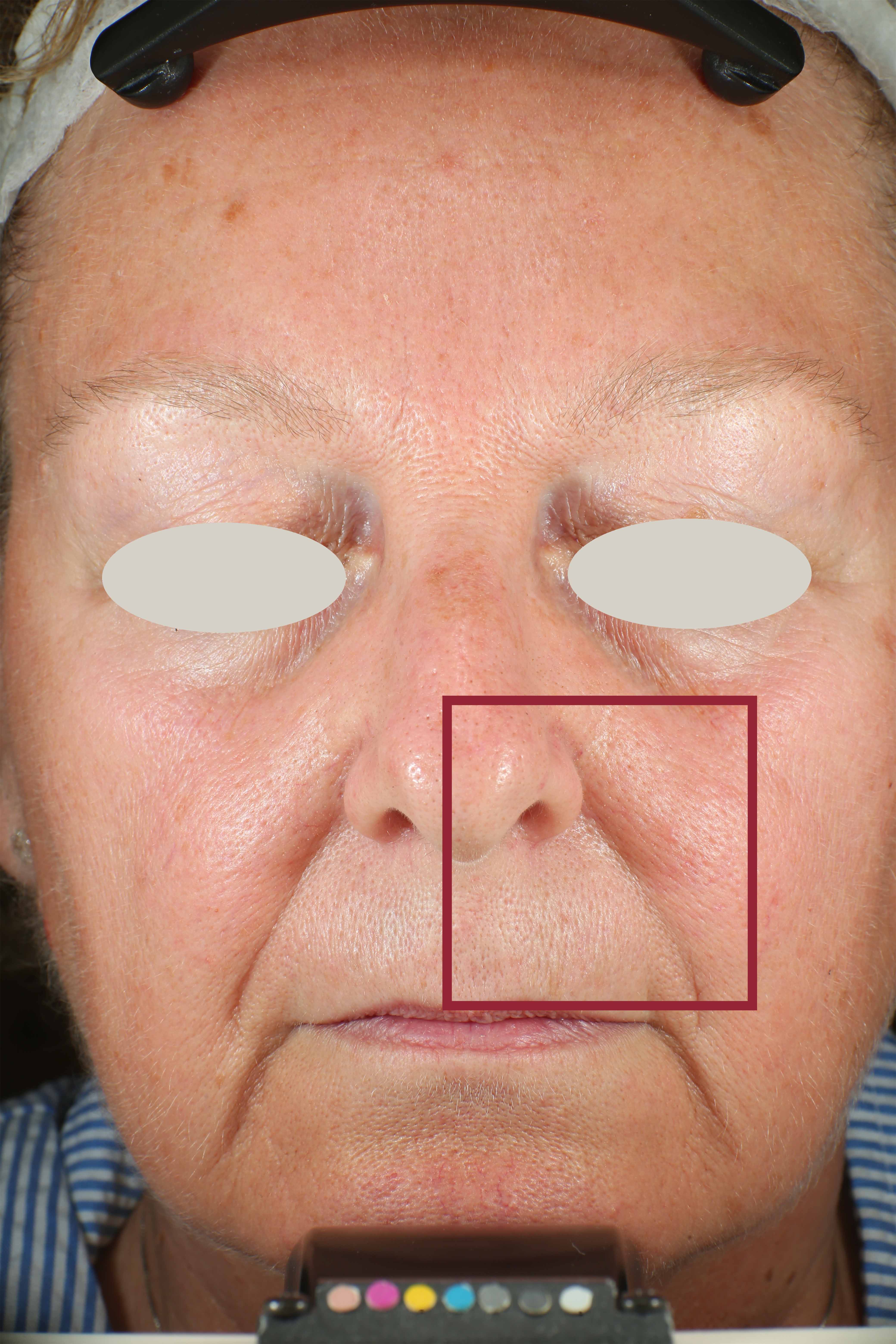
On pictures acquired by Visia®-CR (Canfield Scientific) an image analysis with a dedicated software is carried out in order to evaluate Protoporphyrin distribution. Protoporphyrin is produced by bacteria and a decrease of its distribution can be related to impurities removal.
Protoporphyrin distribution is measured at T0/T4/T8.
Protoporphyrin distribution over the face:

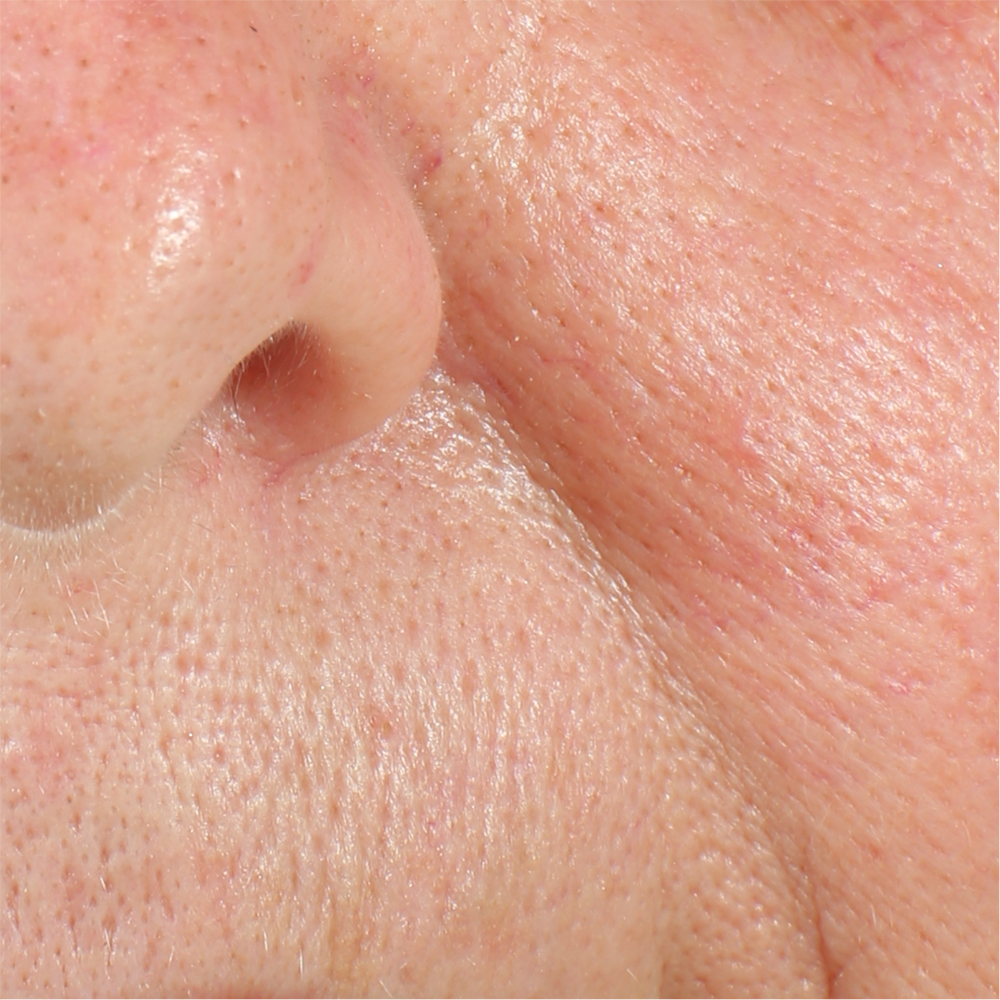
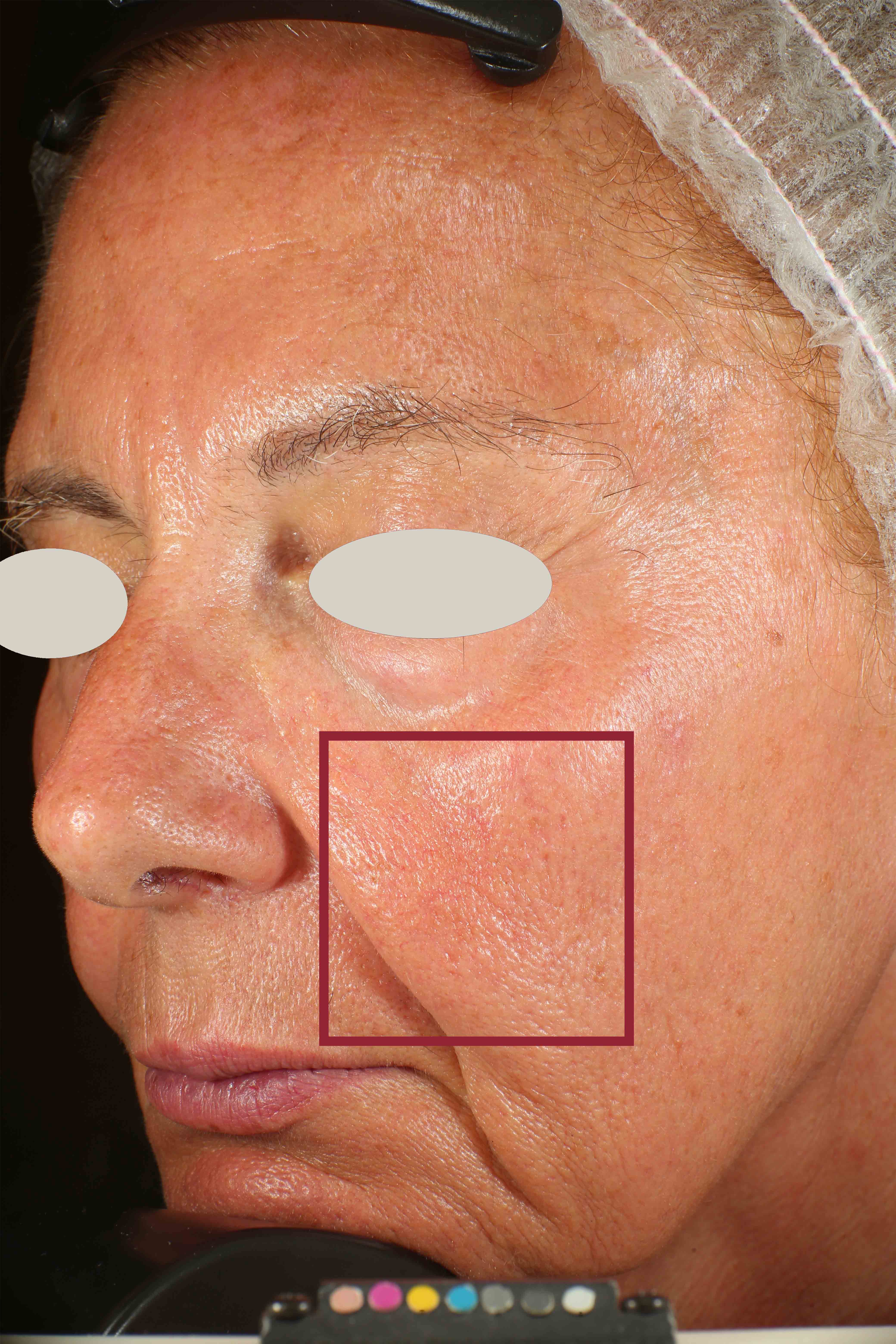
Skin pores reduction
On pictures acquired by Visia®-CR (Canfield Scientific) an image analysis with a dedicated software is carried out in order to evaluate pore dimension (size). For each volunteer, the measurement is carried out in a standardized area of the face.
Pore dimension measured at T0/T4/T8.
Skin microbiome improvement
The skin microbiome analysis is based on the metagenomic analysis of the 16S rRNA gene. 16S rRNA gene was chosen since this gene is present in all bacterial (prokaryotic) cells genetic material, but not in human (eukaryotic) cells. Skin microbiota is collected by brushing a defined skin area with a swab, a non-invasive method allowing to collect the bacteria on the skin surface. The second step of the analysis pipeline consists in isolating the bacterial DNA from other components of the sample (human DNA, sebum, dead skin cells, atmospheric pollutants, etc.). Through a series of cell lysis and extraction steps, the purified bacterial material of a given volunteer is obtained. The bacterial material in each sample is then amplified by PCR in order first to increase the quantity of DNA, and second to label the genetic material in each sample - which will later allow the identification of each sample during the sequencing step. The samples’ concentrations are levelled out prior to pooling, to give the sequencing mix. This solution is then loaded in a cartridge and analysed by a sequencer. At the end of the metagenomic analysis run, the raw files are analysed by means of various dedicated software, and the results compiled according to the client’s requirements.
Analysis performed at T0/T8.
ADDITIONAL SPECIFIC STUDY
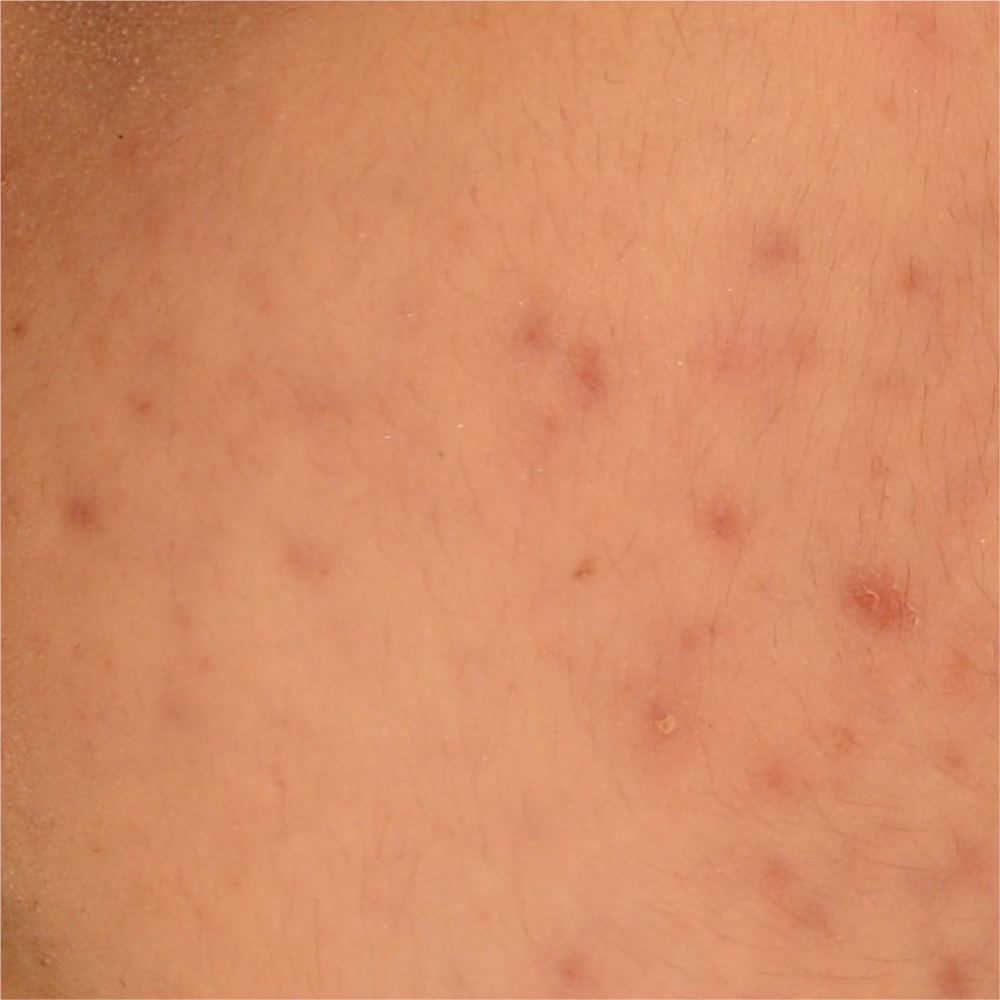
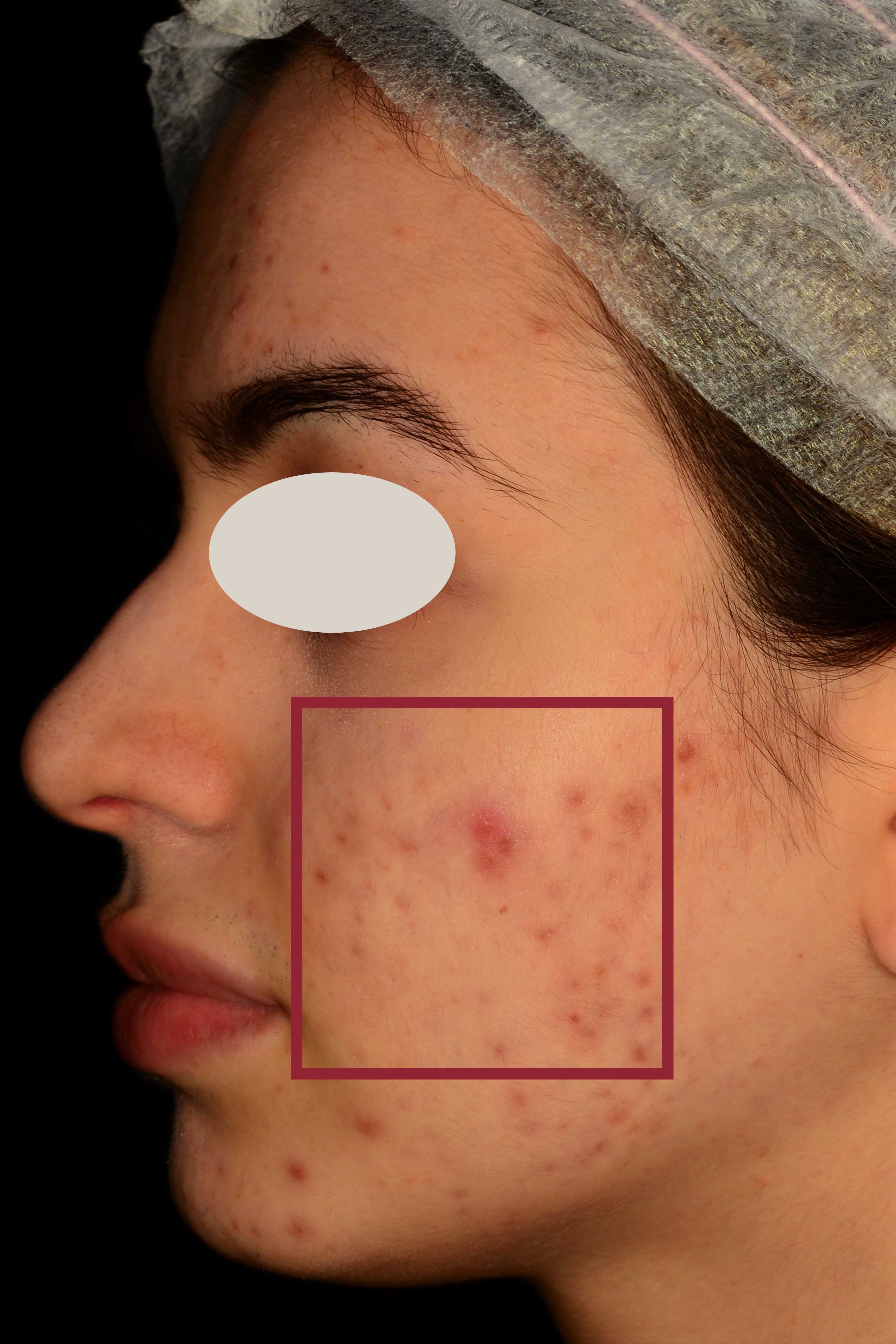
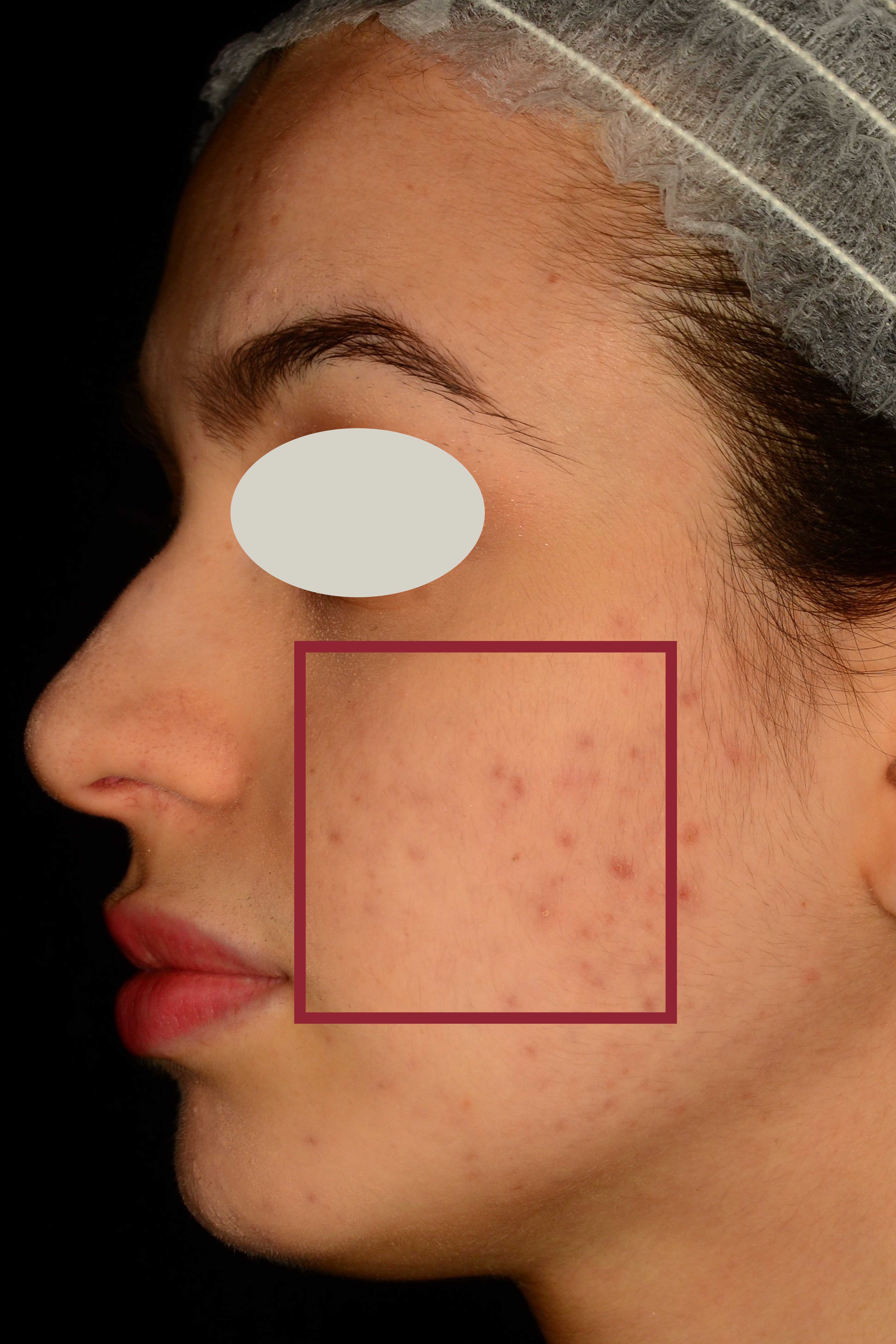
An additional clinical study was conducted to measure the reduction of the number and appearance of acne lesions, and the normalization of the presence of sebum on the skin.
Clinical evaluation of skin complexion evenness and skin inflammatory status of the area interested by acne lesions, clinical evaluation of active acne lesions (inflammatory lesions) and comedones (non-inflammatory lesions), non-invasive bioengineering techniques that allow to evaluate skin moisturization, pH and sebum content.
This additional study aims to explore the synergistic impact of using The Skin Biotic nutraceutical in combination with The Gentle Cleanser compared to a topical product available on the market that is specifically formulated for acne treatment.
SUBJECTS
Participant subjects: 80 female and male subjects aged between 18 and 50 years old (n=20 subjects for each group according to a previously predisposed randomization list) showing acne severity from 1 to 3 according to IGA (Investigator’s Global Assessment) severity scale. The study foresaw 56 days of products use. Instrumental evaluations of the skin parameters under study were carried out at baseline and after 28 days and 56 days of products use.
RESULTS
Evidence-backed outcomes
Transformative results in 8 weeks
Your Questions About AWvi
Why opt for an integrated solution like The Skin Method?
Our vision with The Skin Method was to create an integrated and compact solution tailored to address a multitude of specific skin concerns while ensuring holistic health benefits for every skin type. This objective is achieved through a comprehensive approach that works both from both the inside out and the outside in, leveraging the synergistic actions of our products for exponential benefits. Each of the four products in our launch collection plays a vital role in this system. They are not just individually potent, but also collectively transformative, offering a broad spectrum of benefits from nourishment and balance to protection and regeneration. This curated selection is formulated for all genders, all ages, and all skin types.
What does P.P.EP4151217 correspond to?
P.P.EP4151217 corresponds to the registration number at the European Patent Office (EPO) for the patent of which AWvi is the exclusive owner, aimed at protecting the genuine innovation represented by its solution combining oral and topical formulas. The innovative nature of this solution has undergone thorough verification by the relevant authorities based on extensive clinical tests.
What does medical grade imply?
Products incorporating medical-grade ingredients are meticulously developed and assessed by healthcare experts, utilizing potent actives. This signifies that such formulations undergo rigorous clinical evaluations to affirm their benefits, ensuring they deliver superior outcomes. The comprehensive clinical validation, coupled with the advanced expertise of the professionals involved, assures users of the products' exceptional quality and safety.
For The Skin Biotic, both the manufacturing process and the packaging adhere to stringent protocols inspired from the pharmaceutical industry, guaranteeing the safety, efficacy and longevity of the product.